Heavy metals removal using modified leaves biomass
By: Ahmed Hasham
M.Sc. Env. Analytical Chemistry
Introduction:
Continuous industrial development has resulted in raised levels of toxic heavy metals. This has been entangled, almost everywhere, in most industrial applications involving leakage and redistribution of heavy metals, such as metallurgy, iron and steel, electroplating, leather working etc. Wastewater produced from these industrial activities affect the environment, the human health and ecosystem.1
The heavy metals, such as Hg, Cr, Pb, Zn, Cu, Ni, Cd, As, Co, Sn, etc. must be removed from water to avoid the harmful effect on the environment and human health.
Many methods have been applied for removing metal ions from aqueous solution generally depending on physical, chemical, and biological technologies.2
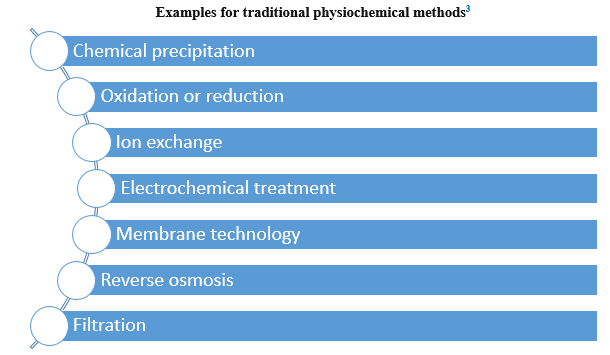
Most of these are ineffective or excessively expensive when the metal concentrations are less than 100 mg/L.
For example, some of these treatment methods will be very costly, especially when treating large amounts of wastewater, so it is became necessary to found an cheap, effective and eco- friendly method to remove heavy metals from water.4
Researches on biosorption focus on the biosorbents, the biosorption mechanism, and large-scale experiments. Although many biological materials can bind heavy metals, only those with sufficiently high metal-binding capacity and selectivity for heavy metals are suitable for use in a full-scale biosorption process. 5
Raw leaves as Biosorbents:
Leaf adsorbents are among the most studied biosorbents for the removal of metal ions, because leaves are considered as adsorbents because it is:
- Available,
- Cheap
- Eco- friendly materials
- The high sorption capacity.6
But, it has been often ignored because it has:
- Low mechanical strength.
So that it must be modified to be avoid this advantage.7
What is the mechanism of metal removal using leaves?
The leaves containing functional groups such as carboxyl, amine, amide, methyl groups and hydroxyl groups which considered the major groups responsible for the biosorption process.8
The pH of the aqueous solution has been considered the most important parameter controlling the metal adsorption by adsorbents. The pH can affect the form and the quantity of metal ions in water and the form and quantity of an adsorbent’s surface sites. In general, the removal of metal cations due to the well-known competition between H ions and metal ions in the solution.9
Modified Leaf Biomass as Heavy Metal Biosorbents:
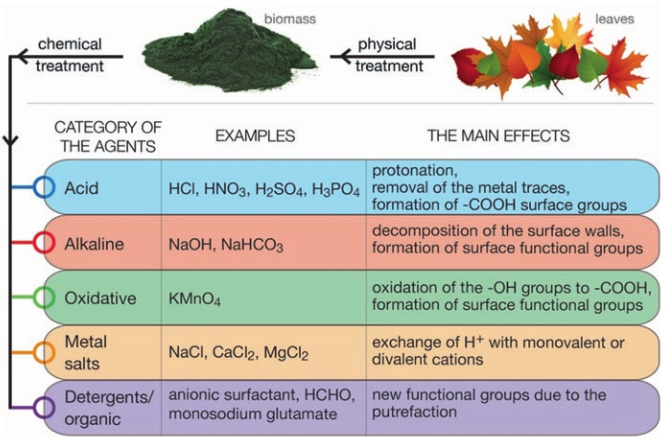
Methods of surface modification:
The main goal of surface modification is to improve the biosorption efficiency. The greatest valuable and widely studied surface modification of leaf biomass is the chemical modification. 10
Advantage of surface chemical modification:
- Low cost
- Procedure is very easy.
- It is a one-step process in the most of the cases.
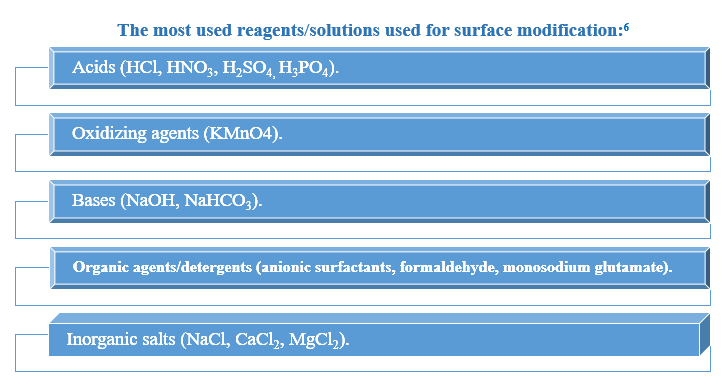
- Classification of surface modification and its aims 6
The use of each modification method aims to a specific effect like to improve the chemical surface heterogeneity, increase the number and spreading of the functional groups available for mandatory with the metal and/or alter the surface morphology; thus the useful pretreatment method should be chosen according to the targeted metal ion.11
Maximum adsorbent capacity an increased with the increase in temperature this is due to the increase in the number of available active sites on the adsorbent.12
The most important factor affected the adsorption performance is the particle size of the biomass powder.
Two different approaches during the developing of the raw biomass was followed:
- To control the particle size of the biomass in a specific range by sieving. The most commonly range was between 250 and 500 μm. 13
- The second one is to collect and use the powder of less than a specific maximum in size value. Different maximum particle sizes were reported, such as 500,180, 100, or even 80 μm.6
Regeneration of biosorbents
The reusability of biosorbents offer an economic benefit and is preferred for the practical and profitable usefulness in wastewater treatment processes. Numerous studies have been done for regeneration and reuse of modified leaf biomass after metal adsorption.8
Desorption studies also help to control the biosorption mechanisms such as ion exchange, complexation and physisorption. The most common eluents used are diluted HCl, NaOH, HNO, and EDTA solutions, usually in concentration up to0.1 mol/L.6
The contact of biosorbents in acidic conditions due to strong desorption agents such as HCl, can affect the biomass rigidity due to biomass degradation and decrease of binding sites number.6,8
References:
Sun, J., Ji, Y., Cai, F., & Li, J. (2012). Heavy Metal Removal Through Biosorptive Pathways. In Advances in Water Treatment and Pollution Prevention (pp. 95-145). Springer, Dordrecht.
Barakat, M. A. (2011). New trends in removing heavy metals from industrial wastewater. Arabian journal of chemistry, 4(4), 361-377.
Fu, F., & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: a review. Journal of environmental management, 92(3), 407-418.
Garg, V. K., Gupta, R., Kumar, R., & Gupta, R. K. (2004). Adsorption of chromium from aqueous solution on treated sawdust. Bioresource technology, 92(1), 79-81.
Volesky, B. (1990). Removal and recovery of heavy metals by biosorption. Biosorption of heavy metals, 7-43.
Kyzas, G. Z., & Kostoglou, M. (2014). Green adsorbents for wastewaters: a critical review. Materials, 7(1), 333-364.
Crini, G., & Lichtfouse, E. (Eds.). (2018). Green Adsorbents for Pollutant Removal: Innovative materials (Vol. 19). Springer.
Ngah, W. W., & Hanafiah, M. A. K. M. (2008). Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresource technology, 99(10), 3935-3948.
Larous, S., Meniai, A. H., & Lehocine, M. B. (2005). Experimental study of the removal of copper from aqueous solutions by adsorption using sawdust. Desalination, 185(1-3), 483-490.
Bai, R. S., & Abraham, T. E. (2002). Studies on enhancement of Cr (VI) biosorption by chemically modified biomass of Rhizopus nigricans. Water Research, 36(5), 1224-1236.
Wang, J., & Chen, C. (2009). Biosorbents for heavy metals removal and their future. Biotechnology advances, 27(2), 195-226.
Babel, S., & Kurniawan, T. A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: a review. Journal of hazardous materials, 97(1-3), 219-243.
Volesky, B. (1990). Removal and recovery of heavy metals by biosorption. Biosorption of heavy metals, 7-43.