By
M. Gamal Khedr
Professor Emeritus, National Research Centre, Cairo
Abstract
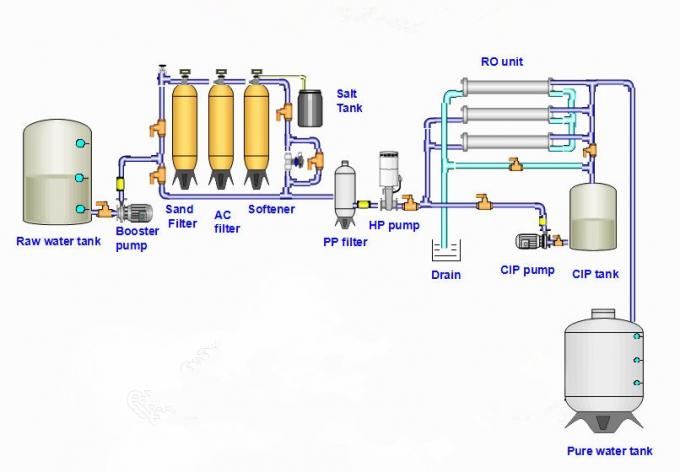
A reverse osmosis (RO) project of 22,000 m3/d was installed for desalination of a groundwater contaminated by the radioactive isotopes radium (Ra 226, 228) to produce drinking water in Qaseem region, Saudi Arabia. The project specified the blending of RO permeate of 20,000 m3/d with 2,000 m3/d of conditioned raw water. Since the radioactive contamination is rather high (195.1 ± 20.2 pCi/L) and it would further increase with time upon withdrawal of groundwater and since, on the other hand, the RO rejection would decrease with time during the membrane lifetime, the project included, as a pretreatment process prior to RO, the partial removal of Ra by adsorption on the active hydrous manganese oxide (HMO) surface and filtration.
However, upon initial start up the plant failed to realize the tender requirements of both lowering of radioactivity up to the maximum contaminant level (MCL) in drinking water of ≤ 5 pCi/L as per the norms of the US Environmental Protection Agency, as well as of rejection of salinity by RO to ≤ 500 mg/L according to the norms of the World Health Organization (WHO).
Our consultancy investigation revealed erroneous application of the technology of Ra removal by adsorption on HMO. This lowered Ra rejection, by this process, to only 57.4% while the project book specified a value of 90%. On the other hand, salt rejection by RO was only 88% instead of 96% as per the project description. These deffects resulted in a product water salinity and Ra contamination out of the requested values upon blending the RO permeate with the conditioned raw water. The consultancy investigation rectified the process of preparation of the active HMO layer which realized the expected Ra adsorption and removal by subsequent filtration. RO performance was also improved by modification of the system design of the RO skids and adopting a developed array according to a standard software projection to obtain salt rejection of 96%. Pilot testing confirmed the satisfaction of the tender conditions.
Keywords: Groundwater Treatment, Radium contamination, Hydrous Manganese Oxide, Preparation of HMO, Adsorption on HMO.
Introduction
Selective membrane processes as RO and nanofiltration (NF) are currently commonly approved as the most efficient and cost effective methods for desalination of brackish groundwater or industrial waste waters of medium salinity (1). On the other hand, RO process was reported to reject efficiently the divalent radioactive ionic species as radium (Ra2+ 226,228) and heavy metal cations (2, 3).
The present project faces the double challenge of a rather high radioactive contamination by Ra 226,228 of 195.1 ± 20.2 pico Curie/L (pCi/L), parallel to too high salinity than suitable for drinking water of 3538.4 mg/L. In view of the similar properties of Ra and Ca, both being alkaline earth metals, consumption of Ra contaminated drinking water would lead to bone deformation, bone cancer and other dangerous attacks then eventual death (4). The present project selected, therefore, the application of RO preceded by a specific pretreatment for partial rejection of radioactivity.
Aim and scope of the project:
- Production of drinking water of total dissolved solids (TDS) ≤ 500mg/L according to the norms of the World Health Organization (WHO) (5).
- Lowering of the Ra radioactivity to ≤ 5pCi/L according to the norms of the US-Environmental Protection Agency (EPA) (6).
- In view of the rather high detected radioactive contamination and the risk of its possible increase with time upon withdrawal of groundwater, and as precaution against the possible decrease of RO rejection during the membrane lifetime, the project book specified partial rejection of Ra by adsorption on the activated MnO2 surface and filtration prior to RO as a double safety barrier against the contamination of drinking water. Hydrous manganese oxide (HMO) on the surface of activated MnO2 is known to adsorb efficiently Ra and other divalent or polyvalent metal cations, under critical specific conditions, and is the basis of a standard method for Ra removal (7).
However, upon the start up of the project neither the specified salt rejection nor the Ra rejection were realized. The present consultancy investigation aims to discover the sources of this failure and propose the adequate rectification for the realization of the project specified performance.
Discussion
Project Facts:
Plant Capacity:
Final product rate of 22,000 m3/d drinking water of which 20,000 m3/d are to be produced by RO then blended with 2,000 m3/d conditioned raw water.
Raw Water Quality:
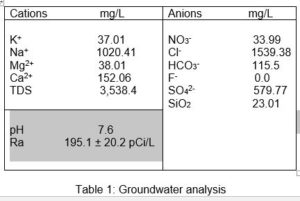
Groundwater analysis is given by Table 1.
The radioactive contamination is due to combined Ra 226,228 of 195.1 ± 20.2 pCi/L.
Tender Described RO System and Performance:
RO membranes of the modern technology of thin film composite, spiral wound membrane elements of the polyamide chemistry (TFC, SW, PA).
Salt rejection of RO units not ˂ 96%
Percent recovery of 85%
Permeate Flux not >20 lmh
Investigation of the Failure of the Partial Removal of Ra:
The project specified the pretreatment of the groundwater for Ra removal by adsorption on freshly prepared surface active layer of HMO then filtration. However, the resultant removal of Ra by this step was quite lower than expected and led to unacceptable contamination of the final drinking water. If we consider the main lines of the procedure of removal of Ra by adsorption on HMO layer:
- MnO2 is prepared according to the reaction:
![]()
- The treatment should start by oxidation of the dissolved iron and manganese from the raw water and filtration for removal of the corresponding oxides to prevent the competitive adsorption of these cations on the HMO layer which lowers Ra adsorption (8).
- Upon oxidation of iron and manganese in the aeration towers a part of Ra is, in fact, removed by adsorption on their deposited oxides (9).
- The reagents MnSO4 and KMnO4 should separately be dissolved in agitated tanks then mixed in a third tank with continuous agitation.
- Since deposition of MnO2 is accompanied by production of H2SO4, an alkali as caustic soda should be dosed to neutralize the pH to the range 8-8.5.
- At this pH, a contact time of 10 to 12hrs with agitation is required for deposition of MnO2 and for its surface hydrolysis.
- Then the resulting suspension is dosed to the feed stream. Usually a dose of 4 mg/L is used.
Analysis of the failure of Ra removal by HMO:
Inspection of the system of preparation of HMO revealed the following defects:
- The above described standard procedure for preparation of the HMO was not respected. The dissolved reagents KMnO4 and MnSO4 instead of being mixed together and left over the mentioned contact time for deposition of MnO2 and surface hydrolysis prior to dosing to the raw water stream, they were directly independently added to the feed water stream.
- These reagents were, therefore, much diluted before reaction without the correct contact time at the suitable pH for the adequate formation of the active layer.
- The added concentrations of these reagents were found to be lower than standard as per equation [1]. According to the operation team this was to prevent the repeated tripping of the plant.
In fact, plant tripping was due to the oxidizing alert caused by the unreacted KMnO4 which raised the oxidation-reduction potential (ORP) in the feed stream above the limit fixed for protection of the PA membranes. The operation decreased the dosing of the oxidizing KMnO4 to avoid the repeated tripping.
These problems explain the incomplete reaction of formation of the HMO layer and consequently the observed failure of Ra removal.
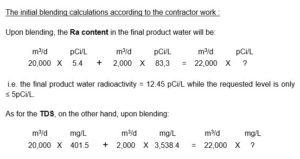
While the process flow diagram of the project tender specified 90% Ra rejection, the feed water radioactivity of 195.1 ± 20.2 pCi/L was lowered to 83.3 ± 8.9 pCi/L i.e. by only 57.4%.
Investigation of the failure of RO units to realize the tender requirements of salt and radioisotope rejection.
The project four (4) RO skids of 5,000 m3/d each were based on 8 inch, TFC/SW/PA membranes according to an RO system design that enabled rejection of TDS to 401.5 mg/L i.e. by only 88,27 % and Ra rejection to 5.4 ± 1.3 pCi/L while the project specified RO salt rejection of ≥ 96%.
The consultant investigation had to upgrade Ra rejection from the feed water by correct preparation of the active adsorbing HMO layer in order to enable safe values of radioactivity upon blending with the RO permeate.
When the preparation of the active layer was rectified according to the described standard procedure, a 92.6 % rejection of Ra was realized to give a residual activity in the RO feed of only 14,48 pCi/L.
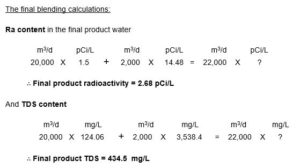
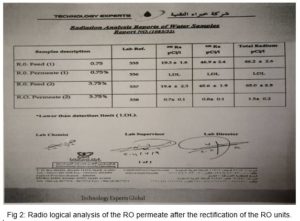
As for RO Rejection, on the other hand, we have developed, based on RO system design projections using a standard software, a different skid design Fig 1 which resulted in permeate TDS of 124.06 i.e. at 96.5 % salt rejection and practically complete Ra the rectified rejection (for blending calculations we have selected the highest reading), Fig 2.
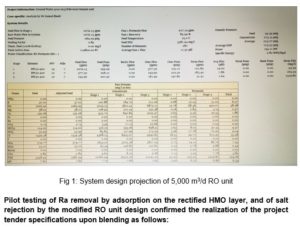
Pilot testing of Ra removal by adsorption on the rectified HMO layer, and of salt rejection by the modified RO unit design confirmed the realization of the project tender specifications upon blending as follows:
Conclusions
- A consultancy investigation was conducted for a case of failure of an RO plant which treats groundwater contaminated by the radioactive isotopes Ra 226,228 at an activity quite higher than the permissible MCL in drinking water. The primary inspection revealed that both TDS and radioactivity of the final product water unacceptably exceed the project specifications.
- While selective membrane techniques as RO or NF show efficient rejection of divalent and polyvalent ionic species as radioisotopes and heavy metal cations, in case of high concentrations of such dangerous contaminants RO can be successfully combined with other decontamination processes as a double safety barrier to realize drinking water norms.
- The pretreatment adsorption of Ra on the adequately prepared active HMO and filtration could realize > 90 % removal of Ra from raw water. Preparation includes mixing of the reagents KMnO4 and MnSO4 with agitation and pH adjustment to the range (8-8.5) over a contact time of 10 hours then dosing to the main stream.
- The Ra bind to HMO in the back wash stream of the media filters together with the RO reject stream go to the evaporation ponds where the radioactivity is diluted in the solid state by the rejected salts and goes to the adequate disposal.
References
- Gamal Khedr, “Optimization of reverse osmosis process efficiency and Environmental safety through reject processing”, Euromembrane International Conference, Hambourg (2004) 600.
- Gamal Khedr, “Nanofiltration and low energy reverse osmosis for rejection of radioactive isotopes and heavy metal cations from drinking water sources”, Desalination and Water Treatment, 2 (2009) 342 – 350.
- J. Sorg et al, “Removal of Ra 226 from Sarasota County, Flo., drinking water by reverse osmosis”, J. Am. Water Works Assoc., 72 (4) (1980) 230.
- M. Finkelsteing and N. Kreiger, “Radium in drinking water and risk of bone cancer in Ontario youths: a second study and combined analysis”, Occup. Environ. Med., 53 (5) (1996) 305 – 11.
- Drinking-Water Quality Guidelines-WHO, https://www.who.int>guidelines 4th edition (2017).
- The Radionuclides Final Rule, Environmental Protection Agency, https://www.epa.gov.>dwreginfo>radio December 2000.
- A. Clifford, “Radon, Radium and Uranium in Drinking Water”, C.R. Cothern & A.P. Rebers, Eds., Lewis Publishers, (1991) 234
- L. Brink et al, “Radium removal efficiencies in Water Treatment Processes”, J. Am. Water Works Assoc., 70 (1) (1978) 31.
- Clifford et al, “Evaluating various adsorbents and membranes for removing radium from groundwater”, J. Am Water Works Assoc., (1988) 94 – 104.