NEUTRALIZATION
Neutralization is a common practice in wastewater treatment and waste stabilization.
If a waste stream is found to be hazardous because of corrosively, neutralization is the primary treatment used. Moreover, neutralization is used as a pretreatment system before a variety of biological, chemical, and physical treatment processes. Since many chemical treatment processes, such as metal precipitation, coagulation, phosphorus precipitation, and water softening are pH dependent, the pH of these processes is adjusted to achieve maximum process efficiency. Furthermore, the pH of the effluent wastewater from different industrial activities also requires adjustment prior to its discharge into receiving water bodies. The US EPA has set pH standards for different types of water; for example, the pH range required to protect marine aquatic life is 5–9. [1]
Neutralization is the process of adjusting the pH of water through the addition of an acid or a base, depending on the target pH and process requirements. Some processes such as boiler operations and drinking water standards need neutral water at a pH of 7.
Water or wastewater is generally considered adequately neutralized if (1) its damage to metals, concrete, or other materials is minimal; (2) it has little effect on fish and aquatic life; (3) it has no effect on biological matter (i.e., biological treatment systems).
In chemical industrial treatment, neutralization of excess alkalinity or acidity is often required. One of the critical items in neutralizing the water is to determine the nature of the substances that cause acidity and alkalinity. This is generally achieved in laboratory scale experiments by preparing titration curves showing the quantity of alkaline or acidic material necessary to adjust the pH of the target wastewater. The nature of titration curves obtained in these experiments is critical in determining the proper chemical type and dose. Methods used for pH adjustment should be selected on the basis of costs associated with the neutralizing agent and equipment requirements for
dispensing the agent.
In neutralization, several parameters need to be assessed and evaluated before the actual pH adjustment is carried out. These parameters are discussed in the following sections.
- pH
pH is the reference indicator for neutralization. Many chemical processes, such as metal precipitation and water softening, which are involved in neutralization, are pH dependent. pH is the negative logarithm of the H+ ion activity in solution .
pH = −log{H+}
If the ionic strength of the waters is not very high (less than 0.01 M), the activity of hydrogen ions can be replaced with the molar concentration of hydrogen ions, If the ionic strength is high, correction factors using the Debye–Huckel equation or Davies equation can be commonly used [1]
In most practical applications, the pH scale ranges from 1 to 14. In pure water and in the absence of materials other than H+ and OH–, water behaves ideally and activity equals molar concentration. Under these conditions, [H+] equals [OH−] as required by electroneutrality. At 25oC, the ion product of water (Kw = [H+][OH−]) is 10−14.
The process of neutralization is not only limited to bringing the pH to 7; it is invariably used in the processes, where pH adjustment to other than 7 is required depending on the chemical process in question. For example, some processes like biological wastewater treatment require pH to be near neutral, whereas other processes like metal precipitation require pH to be in the alkaline range. Some of the important chemical processes, where pH plays a significant role and where pH adjustment through neutralization is often required, are metal adsorption and biosorption, chemical precipitation, water softening, coagulation, water fluoridation, and water oxidation[2-3] .
- Acidity and Alkalinity
Alkalinity is the capacity of water to neutralize acids, whereas acidity is the capacity of water to neutralize bases. The amount of acid or base to be used in the neutralization process depends upon the respective amount of acidity and alkalinity.
The most important source of both alkalinity and acidity in natural waters is from the carbonate system. However, if the wastewater comes from industrial sources, OH− or H+ is also a major contributory factor to alkalinity or acidity, respectively. For example, water from acid mine drainage contains a large amount of acidity because of the presence of sulfuric acid produced from the oxidation of pyrite. Both acidity and alkalinity are expressed in terms of acid/base equivalents. In water and wastewaters where the predominant ions controlling pH are [H+], [OH−], [HCO3 −], and [CO32−], the forms of alkalinity encountered are hydroxide, carbonate, and bicarbonate. These three forms of alkalinity altogether constitute total alkalinity.
- Buffer Capacity
The word “buffer” stands for the stubbornness against any change. In environmental chemistry, buffers are always defined in the context of pH.
pH buffers are those that resist any changes in solution pH when an acid or a base is added into the solution.
They are very important in chemical neutralization processes. Buffers generally contain a mixture of weak acid and their salts (conjugate base) or weak bases and their conjugate acid. A solution buffered at a particular pH will contain an acid that can react with an externally added base and vice versa. The overall efficiency and chemical cost of the neutralization process depend on the presence of pH buffers in wastewaters.
In natural waters and wastewaters, the buffering capacity arises due to the presence of phosphates, carbonates, and other weak organic acids. The mineral composition of natural waters is regulated by a buffer system involving natural clay minerals such illite and kaolinite. Careful consideration should be given while neutralizing such waters. If the buffering capacity of the water or wastewater to be neutralized is not taken into account, the actual amount of neutralizing chemical required may vary widely and causes operational problems.
- Hardness
Hardness in waters arises from the presence of multivalent metallic cations [4]. The principal hardness-causing cations are calcium, magnesium, ferrous iron, and manganous ions. This parameter is important in water-softening processes. The part of the total hardness that is chemically equivalent to the bicarbonate plus carbonate alkalinities is called carbonate hardness. When both hardness and alkalinity are expressed in mg/L as CaCO3, these two are be related as follows:
When alkalinity < total hardness,
Carbonate hardness (in mg/L) = alkalinity (in mg/L)
When alkalinity > total hardness,
Carbonate hardness (in mg/L) = total hardness (in mg/L)
NEUTRALIZATION PRACTICES
Neutralization can be carried out in either batch or continuous mode. In batch mode, the effluent is retained until its quality meets specifications before release. Several processes can be simultaneously carried out when the process is performed batchwise.
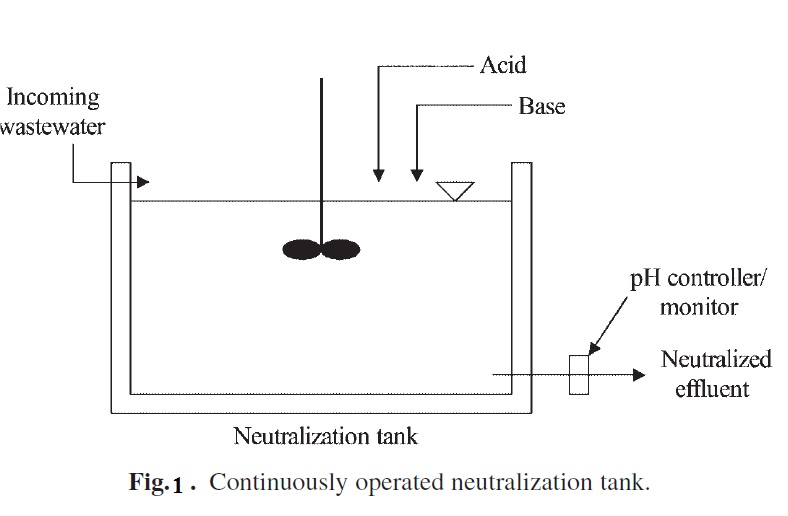
Batch processes are good for small scale treatment plants or small waste volume. For large volumes, a continuous neutralization process is typically used. Figure 6 shows a typical schematic of a continuous neutralization reactor. The use of a batch neutralizing system or continuous flow system depends upon several considerations. In general, continuous flow-through systems are used when
- Influent flow is relatively constant and sudden variations are not expected.
- The influent flow characteristics are essentially constant.
- Effluent chemistry is not very critical. An example is when the process is a part of multistage neutralization process.
Batch neutralization systems are used when:
- There are large fluctuations in influent properties (i.e., flow and pH).
- The influent wastewater contains concentrated acids or bases.
- The effluent quality has stringent discharge limits.
Neutralization tanks should be constructed with a corrosion-resistant material or should be lined to prevent corrosion. Addition of an acid or an alkali should be controlled by continuous pH measurement, either by withdrawing samples periodically and measuring the
pH or by installing an online pH meter that gives continuous pH readings.
- Neutralization of Acidity
The most widely used methods to balance acidity by adding a proper alkaline solution are outlined below (6):
- Mixing alkaline and acidic wastes such that the net effect is nearly neutral pH.
- Passing the acidic water through a limestone bed. This water should not contain limestone- coating substances such as metal salts or sulfuric or hydrofluoric acids.
- Mixing acid waste with lime slurries or dolomitic slurries.
- Supplementing acidic wastewater with proper amounts of caustic soda or soda ash (Na2CO3).
Acidic wastes are neutralized either by adding lime alkalis or by adding sodium alkalis.
The most commonly used lime alkalis are quicklime (CaO) and hydrated or slaked lime (Ca(OH)2) [5–6]. Sodium alkalis involve the use of caustic soda (NaOH) or soda ash (Na2CO3). Calcium and magnesium oxides are considerably less expensive than sodium alkalis and are used more widely (6). Because these oxides are moderately soluble in water, they are typically slurried. Calcium or magnesium alkalis produce more sludge than do sodium alkalis.
Sodium alkali rapidly reacts with acidic wastes and produces soluble neutral salts when combined with most acidic wastewaters. Between the two types of sodium alkalis, caustic soda is a stronger alkali than soda ash. Caustic soda is available in anhydrous form at various concentrations. Soda ash can be purchased as dry granular material.
Liquid caustic soda is produced and supplied in a concentration range of 50–73%. Most industries use a 50% caustic soda solution. The specific gravity ranges from 1.47 to 1.53 depending on the temperature. Caustic soda is very corrosive in nature. Hence all containers
and lines that come in to contact with caustic soda during use or shipment should be carefully selected.
Soda ash, when used as sodium carbonate monohydrate, contains 85.48% sodium carbonate and 14.52% water of crystallization. Hydrated soda ash loses water of crystallization when heated. Heptahydrated and decahydrated are other forms of soda ash used in neutralization practices. Dissolving monohydrated soda ash in water generates heat while heptahydrate and decahydrate absorbs heat in contact with water. Bagged soda ash should not be stored in humid places. Furthermore, excessive air circulation should be avoided. Soda ash contains 99.2% sodium carbonate when shipped.
- Neutralization of Alkalinity
Lowering the pH of a solution is sometimes necessary in some treatment processes or when wastewater is to be discharged in open streams. Discharge of effluent with a pH greater than 8.5 is undesirable and lowering the pH is generally achieved either by adding an acid or by adding carbon dioxide. The process of adding carbon dioxide is called recarbonation and is often practiced in industrial wastewater neutralization. The commonly used acids for pH adjustment of alkaline wastewaters are sulfuric acid (H2SO4), hydrochloric acid (HCl), and nitric acid (HNO3). Among them, sulfuric acid is the most widely used neutralizing agent. Use of nitric acid is restricted because of more stringent nutrient effluent limitations. There is no direct relationship between pH and alkalinity.
Hence, titration curves should be established in laboratories before the design of an alkaline wastewater neutralization system. Sulfuric acid used in wastewater treatment could be 77.7% concentration or 97% concentration with an approximate specific gravity of 1.83 [7,8,9]. Sulfuric acid releases a significant amount of heat when added to water. Precautionary measures must be taken to avoid any chemical accident due to the heat generated when practicing neutralization with sulfuric acid. Hydrochloric acid has an average specific gravity of 1.17 and an acid content of 33% by weight. Properly lined tanks should be used to store this classification of hydrochloric acid. Generally polyvinyl chloride tanks or lined steel tanks are used.
By
Ahmed Ahmed Elserwy
Water & Environmental Consultant
References
[1] W. Stumm and J. J. Morgan, Aquatic Chemistry, John Wiley and Sons, New York, 1981.
[2] J. P. Chen and L. Wang, Characterization of a Ca-alginate based ion exchange resin and its applications in lead, copper and zinc removal. Separation Science and Technology, 36(16),3617–3637 (2001).
[3] F. N. Kemmer, The Nalco Water Handbook, McGraw-Hill, New York, 1988.
[4] L. K. Wang, Y. T. Hung, and N. S. Shammas (eds.), Physicochemical Treatment Processes. Humana Press, Totowa, NJ (2005).
[5]M. L. Davis and D. A. Cornwell, Introduction to Environmental Engineering, 3rd ed., McGraw-Hill, New York, 1998.
[6] C. A. Hazen and J. I. Myers, Neutralization tactics for acidic industrial wastewater. In: Process ngineering for Pollution Control and Waste Minimization (D. L. Wise, ed.), Marcel Dekker, New York, 1994.
[7] US EPA, An Appraisal of Neutralization Processes to Treat Coal Mine Drainage. EPA- 670/2-73-093, U.S. Environmental Protection Agency, Washington, DC, 1973.
[8] US EPA, Design Manual—Neutralization of Acid Mine Drainage, U.S. Environmental Protection Agency, Municipal Environmental Research Laboratory, EPA-600/2-83-001, U.S. Environmental Protection Agency Technology, Cincinnati, OH, 1983.
[9] US EPA, Evaluation of Flow Equalization at a Small Wastewater Treatment Plant, US Environmental Protection Agency, Municipal Environmental Research Laboratory, EPA- 600/2-76-181, U.S. Environmental Protection Agency, Cincinnati, OH, 1976.