Adjusting the pH of water and wastewater is carried out by the addition of an acid or a base, depending on the target pH and process requirements. Some processes such as boiler operations and drinking water standards need neutral water at a pH of 7.
Water or wastewater is generally considered adequately neutralized if (1) its damage to metals, concrete, or other materials is minimal; (2) it has little effect on fish and aquatic life; (3) it has no effect on biological matter (i.e., biological treatment systems).
In chemical industrial treatment, neutralization of excess alkalinity or acidity is often required. One of the critical items in neutralizing the water is to determine the nature of the substances that cause acidity and alkalinity. This is generally achieved in laboratory scale experiments by preparing titration curves showing the quantity of alkaline or acidic material necessary to adjust the pH of the target wastewater. The nature of titration curves obtained in these experiments is critical in determining the proper chemical type and dose. Methods used for pH adjustment should be selected on the basis of costs associated with the neutralizing agent and equipment requirements for dispensing the agent. [1]
Common Neutralization Treatments in industry
The application of neutralization varies from industry to industry. The most common application includes neutralization of acidic waste from mining industries, in chemical precipitation, water softening, and wastewater coming out from electronic manufacturing plants, and coagulation and flocculation in wastewater-treatment plants. Neutralization is also required for treated wastewater if the pH of such water is found to be higher or lower than the permissible discharge limits. The following application is for neutralization and pH adjustment in industrial field.
- Water Softening
As explained earlier, hardness of water is caused by the presence of polyvalent metal cations. The major disadvantages of using this type of water are the increased consumption of soap required to produce lather when bathing or washing clothes and the formation of scales in boilers if this hard water is used for generating steam. Chemical precipitation is commonly employed to soften the water, where alkalis are added to the water to raise the pH and precipitate the metal ions in the forms of hydroxides and carbonates.
The softened waters usually have high pH values in the range of 10.5 and are supersaturated
with calcium carbonate and magnesium hydroxide. For further use of such high pH waters, acid neutralization is applied. Adjustment of pH toward neutrality is accomplished either by recarbonation or by adding sulfuric acid [1] .
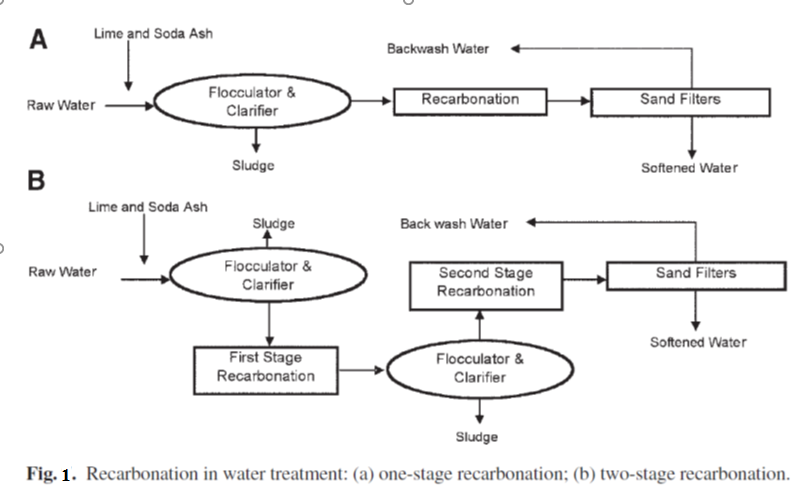
pH adjustment by recarbonation can proceed in two different ways: one-stage recarbonation or two-stage recarbonation. In one-stage recarbonation, enough CO2 is passed only one time to drop the pH to the desired level. When sulfuric acid is used in place of CO2 in one-stage recarbonation, the process is simply called one-stage neutralization. In two-stage recarbonation, CO2 is added to water at two different points after excess lime treatment. At the first point of addition, the CO2 is passed to precipitate calcium carbonate.
In the next step, CO2 is added to adjust pH to acceptable levels. Figure 1 shows a schematic of one-stage and two-stage recarbonation.
- Metal Precipitation
Metal precipitation through formation of metal hydroxide is one of the common methods of metal removal in industries. At high pH, most of the metal hydroxides are insoluble and come out of the solution in the form of metal hydroxide precipitates.
Metals are precipitated as the hydroxides through the addition of lime or another base to raise pH to an optimum value [2, 3, and 4]. Metal carbonate precipitates can also be formed once soluble carbonate solutions such as sodium carbonate are added into metal solutions. Because pH is the most important parameter in precipitation, control of pH is crucial to the success of the process.
- Mine Drainage
The wastewater coming out of mining industries is highly acidic due to the presence of sulfuric acid in appreciable quantities. Acid water coming out of mining industries is one of the common problems prevalent in United States and around the world. Sulfide minerals, mainly pyrite (FeS2), which are often present in mine waste, can generate acid mine drainage when the waste comes in contact with water and air. Pyrite oxidizes to release sulfuric acid into water, resulting in a pH decrease that can be lower than 2. Most of the metals coming in the mine waste dissolve at this pH, resulting in water that is toxic to aquatic life. Chemical treatment by neutralization and subsequent precipitation is often applied to acid mine drainage. The pH range for point source discharge set by the US EPA.
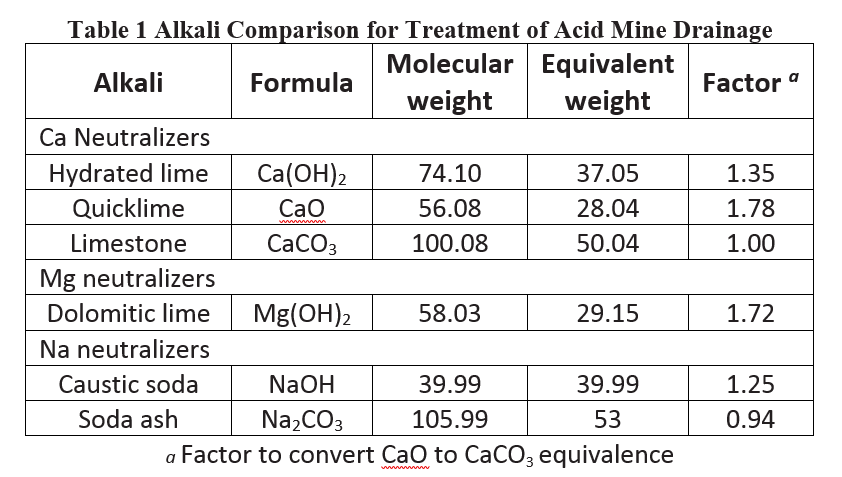
is in the range of 6–9. The alkali comparison for acid mine drainage is given in Table 1 [5].
- Metal Sorption
Activated carbons have successfully been used for metal removal [6, 7]. They are normally used for filtration of suspended solids, as well as adsorption of both organic and metal substances. Removal of heavy metal ions from waste streams by inexpensive recyclable biosorbents has emerged as an innovative technique in the last two decades [8, 9]. The major advantage is the high removal efficiencies for metal ions. This process is normally termed as biosorption. Normally biodegradation isn’t involved as most biosorbents are inactive. The term “biosorption” is used simply because the biosorbents are made from organisms, such as bacteria and seaweed.
Numerous studies have shown that the sorption of metal ions from aqueous solutions is strongly pH dependent. An increase of the solution pH results in a decrease of positive surface charge and an increase of negatively charged sites and, eventually, an increase of metal ion binding. Normally the pH effect becomes less important when the pH is above 4–6. The metal ion adsorption onto activated carbon increases from 5% to 99% from pH 2.0 to 5.5 [6,7]. Sorption experiments using calcium alginate beads (a biosorbent) demonstrated that the metal removal percentages increased from 0 to almost 100% (for metal concentrations < 0.1 mM) from pH 1.2 to 4 and a plateau was established at a pH > 4 [8]. Therefore, neutralization pretreatment must be performed if the initial pH value of metal waste stream is less than 6.
pH Adjustment for industrial wastewater [10]
Unlike domestic or municipal sewage where the pH range is typically 6.0∼7.5, industrial wastewaters have pHs which vary over a much broader range — from very acidic to very alkaline. It should also be noted that a factory may generate a number of wastewater streams and among these can be those which are acidic while the rest may be alkaline.
Consequently it can be useful in terms of reducing chemical consumption for pH adjustment by providing sufficient equalization prior to pH correction so that the various wastewater streams may achieve a degree of pH adjustment through their own interaction. This becomes particularly important if the acidic and alkaline streams are not generated at the same time. Holding and blending becomes a necessary activity then. The difficulties with pH need not always occur because it is an inherent characteristic of the wastewater. It should be noted that pH may be manipulated if chemical cracking of oily emulsions and coagulation had been necessary and may subsequently need to be adjusted again prior to biological treatment. Automatic pH correction can be an unexpectedly difficult activity to perform satisfactorily. This is, in part, because of the difficulty associated with mixing a small quantity of reagent uniformly with a large volume of wastewater. This is made even more difficult if wastewater characteristics, such as its flow rate, changes rapidly. The value of adequate equalization or blending prior to pH adjustment cannot be overstated.
Because of the relatively small size of many industrial wastewater treatment plants IWTPs the preferred chemical for pH adjustment of acidic wastewaters is usually sodium hydroxide instead of lime. A solution of sodium hydroxide would be prepared prior to its injection into the pH correction tank. At IWTPs where the chemical consumption is sufficiently large to justify the additional handling facilities required, lime made up in the form of a slurry can be used. The handling of lime powder (its typical form when delivered to the IWTP) has safety requirements which operators at small IWTPs may not be equipped to cope with. Lime is usually chosen because it is cheaper than sodium hydroxide. When lime is used, it is necessary to appreciate that it is slower compared to sodium hydroxide. This means that the reaction tank has to be increased in size to allow for the longer hydraulic retention times needed.
Typically a minimum of 20 min HRT is allowed for. The reaction tank’s contents are mixed either with a mechanical stirrer or with air.
Where alkaline wastewaters need to be pH corrected, the chemical frequently used is sulphuric acid. The reason for the choice is again usually cost. If the downstream processes include an anaerobic process and relatively large quantities of acid are required, then sulphuric acid may be substituted with hydrochloric acid. This is because the sulphates can be reduced in the anaerobic process resulting in odorous and corrosive hydrogen sulphide being released with the gaseous emissions from the anaerobic reactor.
An example of pH correction station at a pharmaceutical factory. [10]
Two-chambered pH correction station designed to deal with the high pH of the incoming wastewater. The latter had been 9.5 and higher on several occasions. It should be noted that the relationship between pH and the reagent flow required to bring about change is highly nonlinear.
This arises because of the logarithmic nature of the pH scale. A change of one pH unit means a ten-fold change in acidity or alkalinity. The tank’s two chambers were arranged in series with the first smaller than the second. Two stage pH adjustments was practiced with pH in the first chamber being adjusted over a relatively broad band with a larger dosing pump while pH adjustment in the second chamber was over a much narrower band with a smaller dosing pump. This approach was necessary to avoid the swings in pH values if a single adjustment chamber with a large dosing pump had been used.
The vessels used for the various unit processes in an IWTP may be built as fully excavated, partially excavated, or at ground level. Often the decision as to which level a vessel should be placed depends on the desired hydraulic grade line so that flow through an IWTP can be maintained, insofar as is practicable, without the aid of further pumping after the lift at the start of the plant. While the preceding may indeed be a frequent criterion for deciding the level, it need not always be so.
The pH correction station had excavated vessels. The primary reason for doing so in this instance was to maintain a level of aesthetics acceptable to the owner.
Many IWTPs include a bioprocess in their treatment train. Bioprocesses are sensitive to pH conditions and operate well within the fairly narrow range of 6.5∼7.5.While pH conditions outside of this range need not necessarily result in a toxic condition, the bioprocess may nevertheless be inhibited or certain species of micro-organisms may be favored over the more desirable species. One of possible consequences of the latter phenomenon is bulking sludge. Failure to adequately control pH has been noted to be the cause of a surprisingly large number of IWTPs failing to produce wastewater of the required quality from both the bioprocesses and physico-chemical processes.
By
Ahmed Ahmed Elserwy
Water & Environmental Consultant
References
[1] L. K. Wang, Y. T. Hung, and N. S. Shammas (eds.), Physicochemical Treatment Processes. Humana Press, Totowa, NJ (2005).
[2] W. Stumm and J. J. Morgan, Aquatic Chemistry, John Wiley and Sons, New York, 1981.
[3] J. P. Chen and H. Yu, Lead removal from synthetic wastewater by crystallization in a fluidizedbed
reactor, Journal of Environmental Science and Health, Part A-Toxic/Hazardous Substances & Environmental Engineering, A35(6), 817–835 (2000).
[4]. L. K. Wang, Y. T. Hung, H. H. Lo, and C. Yapijakis (eds.), Handbook of Industrial and Hazardous Wastes Treatment. Marcel Dekker, Inc., NY, NY. (2004).
[5]. US EPA, Design Manual—Neutralization of Acid Mine Drainage, U.S. Environmental Protection Agency, Municipal Environmental Research Laboratory, EPA-600/2-83-001, U.S. Environmental Protection Agency Technology, Cincinnati, OH, 1983.
[6]. J. P. Chen and S. N. Wu, Acid/base treated activated carbons: characterization of functional group and metal adsorptive properties, Langmuir, 20(6), 2233–2242 (2004).
[7]. J. P. Chen and S. N. Wu, Study on EDTA-chelated copper adsorption by granular activated carbon, Journal of Chemical Technology and Biotechnology, 75(9), 791–797 (2000).
[8]. J. P. Chen and L. Wang, Characterization of a Ca-alginate based ion exchange resin and its applications in lead, copper and zinc removal. Separation Science and Technology, 36(16),3617–3637 (2001).
[9]. J. P. Chen, L. Hong, S. N. Wu, and L. Wang, Elucidation of interactions between metal ions and Ca-alginate based ion exchange resin by spectroscopic analysis and modeling simulation,Langmuir, 18(24), 9413–9421 (2002).
[10] Industrial Wastewater Treatment ,NG Wun Jern ,National University of Singapore , Imperial College Press 2006, p (54-56).