Coagulation
The principal units of chemical treatment of industrial wastewater that are Coagulation, flocculation and precipitation.
Coagulation is a destabilization process by particle charge neutralization and initial aggregation of colloidal and finely divided suspended matter by inorganic coagulant. To accelerate this process, a coagulant aid (often a high molecular weight cationic polymer) may be used.
The colloids commonly found in wastewater are stable because of the electrical charge that they carry. The charge of colloids can be positive or negative. However, most colloidal particles in wastewater have a negative charge [1].
In addition, coagulation can also produce the removal of particles larger than colloidal particles due to the entrapment of such particles in the flocs formed during coagulation. In most water treatment plants, the minimal coagulant concentration and the residual turbidity of the water are determined by the Jar-Test technique. Besides, physicochemical treatment allows reducing dissolved, suspended, colloidal and non settable matter as well as coloring from dyes. Coagulation or flocculation process was conducted for the treatment of industrial wastewater to achieve maximum removal of COD, BOD and TSS [2], [3].
The optimum dose of a coagulant or flocculent is defined as the value above which there is no significant difference in the increase in removal efficiency with a further addition of coagulant or flocculent.
Coagulation takes place in rapid mix or flash mix basin they are very rapid. The primary function of rapid mix is to disperse the coagulant so that it contacts all of the wastewater.
Coagulants and coagulants aids
Coagulants, i.e., chemicals that are added to the water to achieve coagulation, should have the following three properties:
- Trivalent metallic cations or polymers whose effectiveness as coagulants has been determined.
- Nontoxic and without adverse physiological effects on human health.
- 3. Insoluble or low solubility in the pH ranges common in water-treatment practice. This is necessary in order to have an efficient coagulation process and to be able to leave the lowest possible residual of the chemical in the treated water. [4]
There are many chemicals and polymers that are used in coagulation and flocculation to treat wastewater. The chemicals are used either alone or with various other coagulant aids to promote settling of suspended solids in wastewater. Choices of specific coagulants and coagulants aids depend on the nature of the solid-liquid system to be separated. Salt content and pH affect the surface charges of suspended solids. The sign, magnitudes, and distribution of these surface charges strongly influenced the type and quantity of coagulant to be used.
Polymers as coagulants aids
Polymers are frequently used in conjunction with metal salts to aid in the coagulation process [5].polymers facilitate the use of lower dose of metal salts , enhance floc formation , improve settling efficiency ,increase overflow rate and reduce sludge production .
Polymers may be divided into categories: natural and synthetics .important natural polymer includes polymers of biological origin and those are derived from starch products, cellulose derivatives, and alginates. Synthetic polyelectrolyte consists of simple monomers that are polymerized into high molecular weight substances. Polymer are classified as anionic (negatively charged), cationic (positively charged) or uncharged (nonionic).
There are three different methods to destabilize colloidal particles. First, polyelectrolyte acts as coagulant lowering the charges of the wastewater particles. Cationic polyelectrolyte is usually used for wastewater since wastewater particles are normally negatively charge.
Accurate and precise control of dosage is very important for feeding of polymers in treatment plants. There is a narrow range for maximum performance. Concentrations lower than necessary will not produce effective coagulation, whereas over dosing of polymers will results in charge reversal and restabilization of the colloidal system. Also polymers are more expensive compared to metallic salts. However, this is usually more than compensated for by the lower polymer dosage as well as the reduced sludge production [6].
- Polyacrylamides
Polyacrylamide (PAM) is a commonly used as polymeric flocculent because it is possible to synthesize polyacrylamides (PAMs) with various functionalities (positive, neutral and negative charge) which can be used to produce a good settling performance at relatively low cost. The advantage of polymeric flocculants is their ability to produce large, dense, compact and stronger flocs with good settling characteristic compared to those obtained by coagulation.
Nonionic PAM is used as a flocculent in solid-liquid separations, usually as an aid to primary coagulants such as aluminum and iron salts. The majority of anionic PAM is used in water treatment and industrial wastewater treatment [7].
Furthermore, the polymer performance is less dependent on pH. There are no residual or metal ions added such as Al3+ and Fe3+, and the alkalinity is maintained.
The flocculation performance of flocculants primarily lies on the type of flocculant and its molecular weight, ionic nature and content, on the suspension content in the wastewater and the type of wastewater [7].
- Polyaluminum Chloride (PAC)
Polyaluminum Chloride is PAC for short. It is a kind of water-soluble inorganic polymers with high molecular weight. The structural formula is [AL2(OH) nCL6– nLm], in which the (m) represents the degree of polymerization.
Polyaluminum chloride (PAC) coagulant has been developed and used in water and wastewater treatment since 1980s throughout the world [8].
In recent years, much attention has been paid to hydrolyzing metal salt coagulants namely polyaluminum chloride due to its higher coagulant efficiency and relative low cost compared to the conventional coagulants [9-10].
Besides, PAC poses a good structure and higher charge density which leads to decrease in dosage requirements and hence lesser sludge production [11].
The application of PAC as a coagulant for the removal of color, COD and ammonia from water and wastewater has been investigated by several researchers, [12-13].
Polyaluminum Chloride has high treatment efficiency for Suspended solids, heavy metals, and chemical oxygen demand (COD), and a superior performance at low water temperature in water and waste-water treatment. [14].
PAC has been found by others to be an acceptable alternative flocculating and coagulating agent for drinking water, wastewater, and industrial water treatment [15,16].
Role of polymers in Interparticle bridging
Since synthetic polymeric compounds have large molecular sizes and multiple electrical charges along a molecular chain of carbon atoms, they are effective for the destabilization of colloids in water.
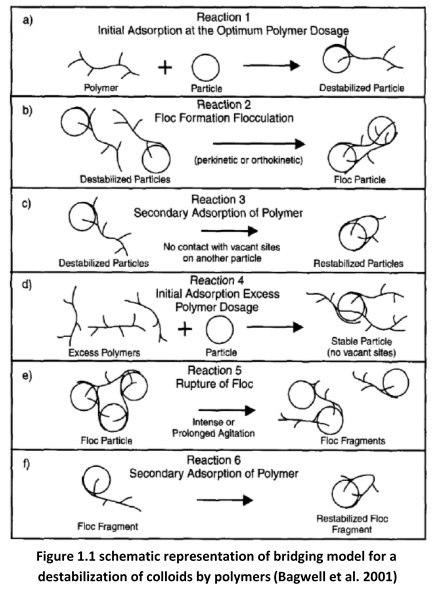
The interparticle bridging process was summarized by [17]. As follows.
Figure 1.1 a shows the simplest form of bridging, a polymer molecule will attach to a
colloidal particle at one or more sites. Colloidal attachment is caused by coulombic attraction if the charges are of opposite charge or from ion exchange, hydrogen bonding, or vander Waal’s forces.
Figure 1.1b shows the second reaction, in which the remaining length of the polymer molecule from the colloidal particle in the first reaction extends out into the solution.
Attachment can occur to form a bridge if a second particle having some vacant adsorption sites contacts the extended polymer molecule. Thus, the polymer serves as the bridge. However, if the extended polymer molecule does not contact another particle, it can fold back on itself and adsorb on the surface of itself, as shown in Figure 1.1c. The original particle is restabilized.
If the quantity of polymer is overdosed, polymer segment may saturate the colloidal surfaces, thus no sites on the surfaces are available for interparticle bridging. This reaction (Figure 1.1d) causes restabilization of the particles. Intense agitation in solution can cause restabilization because polymer-surface bonds or bridges formed are destroyed. These reactions are shown in Figure 1.1e and 1.1f respectively.
Cationic polymers can be effective in coagulating negatively charged clay particles; they do not require a large molecular weight to be effective in destabilization. Electrostatic forces or ion exchange is the process by which the polymers become attached to the clay particles. In general, cationic polymers assist in particle destabilization by charge neutralization and therefore assist in color and suspended solids removal [18].
Anionic polymers of large molecular weight or size are able to bridge the energy barrier between two negatively charged particles, thereby effectively enhancing the coagulation efficiency. Generally speaking, anionic polymers can only assist in the physical process of flocculation. Polymers reduce turbidity by inter-particle bridging but do not affect the removal of color. The use of polymers offers a number of benefits. For instance, polymers increase the rate of flocculation, produce larger, denser floc that settles faster and strengthen the floc which helps improve filtration. They enable a greater volume of wastewater to be treated in a given plant size.
By
Ahmed Ahmed Elserwy
Water & Environmental Consultant
References
[1] Koohestanian, A., Hosseini, M. and Abbasian, Z., The Separation Method for Removing of Colloidal Particles from Raw Water. American-Eurasian J. Agric. & Environ. Sci., 4, 266-273(2008).
[2] Abu hassan, M. A., Li, T.P. and noor, Z.Z., coagulation and flocculation treatment of wastewater in textile industry using chitosan. J. Chem. Nat. Resour. Eng., 4, 43-53(2009).
[3] Egyptian Environmental Affairs Agency (EEAA); Egyptian Pollution Abatement Project (EPAP) Inspection Manual of industrial wastewater treatment plants ,January 2003).
[4] Mackenzie, L. D. and D. A. Cornwell, Introduction to Environmental Engineering, PWS Publishers, Boston, MA, 1985.
[5] Binnie, C., Kimber, M. and Smethurst. G., Basic water treatment. Thomas Telford Ltd., London, 2002.
[6] Viessman, W Jr. and Hammer, M. J, Water Supply and Pollution Control, 5th ed., Harper Collins College Publishers, New York, NY, 1993.
[7] Qian J.W, Xiang X.J, Yang W.Y, Wang M, and Zheng B.Q, Flocculation performance of different polyacrylamide and the relation between optimal dose and critical concentration, Eur. Polym. J. 40: 1699–1704, 2004.
[8] Jiang, J. Q., 2001. Development of coagulation theory and pre-polymerized coagulants for water treatment Separation and Purification Methods, 30(1), 127-141.
[9] Hu C., Liu H. and Qu J., Preparation and characterization of polyaluminum chloride containing high content of Al13 and active chlorine, Colloids and Surf. A: Physicochem. Eng. Asp.260, 109-117 (2005).
[10] Wang Y., Gao B.Y., Xu X.M., Xu W.Y. and Xu G., Characterization of floc size, strength and structure in various aluminum coagulants treatment, J. Colloid Interf. Sci., 332, 354-359 (2009).
[11] McCurdy K., Carlson K. and Gregory D., Flocs morphology and cyclic shearing recovery: comparison of alum and polyaluminum chloride coagulants, Water Res., 38, 486– 494 (2004).
[12] Gao B., Hahn H.H. and Hoffmann E., Evaluation of aluminium –silicate polymer composite as a coagulant in water treatment, Water Res., 36, 3573-3581 (2002).
[13] Wang D., Sun W., Xu Y., Tang H. and John G., Speciation stability of inorganic polymer flocculant-PACI, Colloid Surf. Physicochem. Eng. Asp., 243, 1-10 (2004).
[14] Dempsey, B.A., Chemistry of coagulants. Influence of coagulation on the selection, operation, and performance of water treatment facilities and processes, Seminar of the American Water Works Association (AWWA), Denver. Colorado: 19-30, 1987.
[15] ClearTech Industries Inc, Drinking water treatment. Clear PAC-180: 1327-41, 2008.
[16] Alhadidi A, Kennedy M, Diepeveen A, and Prummel H, Scaling potential calculations using different methods. Desalination and Water Treatment 6:138-143, 2009.
[17] Bagwell, T., Henry, H.B. and Kenneth, M.B., Handbook of public water systems. 2nd Edition, HDR Engineering Inc., New York, 2001.
[18] Yan Jin “Use of a high resolution photographic technique for studying coagulation/flocculation in water treatment ” civil and geological engineering department, University of saskatchewan saskatoon, Canada, (2005).