Introduction [1]
Flotation may be used in lieu of the normal clarification by solids-downward-flow sedimentation basins as well as thickening the sludge in lieu of the normal sludge gravity thickening. Water containing solids is clarified and sludges are thickened because of the solids adhering to the rising bubbles of air. The breaking of the bubbles as they emerge at the surface leaves the sludge in a thickened condition.
In a flotation system for solid/liquid separation, there are at least two methods by which gas bubbles can be used to increase the buoyancy of suspended solids: (a) entrapment of the bubbles in the particle structure; and (b) adhesion of the bubbles to the particle surface (see Fig. 1). In the former case, as the gas bubbles rise toward the surface, the controlled turbulence in the inlet compartment causes contact between the solids.
The floc, formed by the natural floc-forming properties of the materials or by the chemicals
that have been added, increases in size because of more contact with other solids.
Eventually, a structure is formed that does not permit rising gas bubbles to pass through or around it. [2]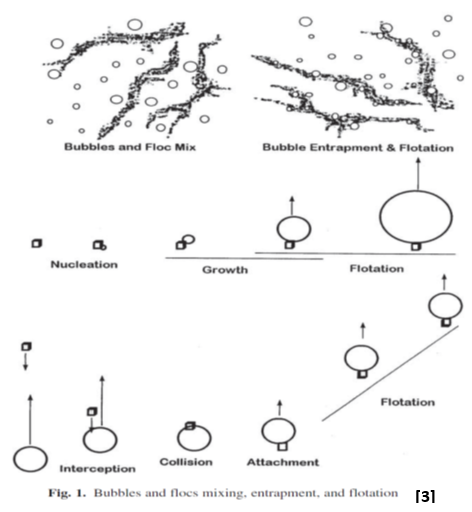
Ozone
Ozone is an oxidant. Ozone (O3), sometimes called “activated oxygen”, or “triatomic oxygen”, contains three atoms of oxygen rather than the two atoms we normally breathe. Ozone is the second most powerful oxidant in the world and can be used to destroy bacteria, viruses, and odors.
Ozone is a gas at ambient temperatures and pressures with a strong odor. Ozone can be produced as a gas from oxygen in air, or concentrated oxygen. This ozone gas can be dissolved into water, or used in the gas phase for a variety of applications.
Several investigators developed an in-depth analysis of the reactions for ozone with various organic compounds. These reactions were described by Miller et al. [4] and are summarized below:
(a) Aromatic compounds: Phenol reacts readily with ozone in aqueous solution. Oxalic and acetic acids are relatively stable to ozonation in the absence of a catalyst such as ultraviolet light or hydrogen peroxide. Cresols and xylenols undergo oxidation with ozone at faster rates than does phenol. Pyrene, phenanthrene, and naphthalene oxidize by ring rupture.Chlorobenzene reacts with ozone slower than does phenol.
(b) Aliphatic compounds: There is no evidence that ozone reacts with saturated aliphatic hydrocarbons under water- or wastewater-treatment conditions. There is no evidence that ozone oxidizes trihalomethanes. Ozone combined with ultraviolet radiation does oxidize chloroform to produce chloride ion, but no identified organic oxidation product. Unsaturated aliphatic or alicyclic compounds react with ozone.
(c) Pesticides: Ozonation of parathion and malathion produces paraoxon and malaoxon, respectively, as intermediates, which are more toxic than are the starting materials.
Continued ozonation degrades the oxons, but requires more ozone than the initial reaction. Ozonation of heptachlor produces a stable product not yet identified. Aldrin and 2,4,5-T are readily oxidized by ozone, but dieldrin, chlordane, lindane, DDT, and endosulfan are only slightly affected by ozone.
(d) Humic acids: Humic materials are resistant to ozonation, requiring long ozonation times to produce small amounts of acetic, oxalic, formic, and terephthalic acids, carbon dioxide, and phenolic compounds. Ozonation of humic materials followed by immediate chlorination (within 8 min) has been shown to reduce trihalomethane formation in some cases. Ozonized organic materials generally are more biodegradable than the starting, unoxidized compounds
Application of Ozone
Ozone acts both as a very strong oxidizing agent and as a very effective disinfectant.
Consequently, it has multiple uses in potable water treatment, wastewater renovation,
cooling water towers, groundwater remediation, and industrial waste treatment. The
following is a snapshot description of each of its applications [5-8].
Dissolved Ozone Flotation (DOF)
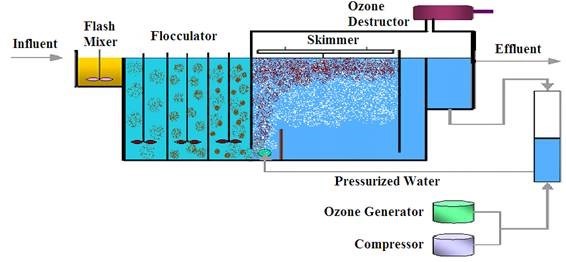
Conventional flotation relies on floating of suspended solids on the top of liquid by air bubbles. A better separation effect is obtained when air bubbles are very small (micro-bubbles and nano- bubbles). In traditional dissolved air flotation (DAF) systems suspended solids and oily compounds are removed by coagulation, flocculation and removing the formed sludge by flotation by applying small air bubbles to increase buoyancy. Dissolved COD remains unaffected in DAF systems. By employing DOF, high concentration of ozone is available, which means a high potential of ozone oxidation and a high volume of micro-bubbles.
Dissolved COD remains unaffected and leaves a DAF with the effluent. When ozone is added to the air bubbles, it partially decomposes to highly reactive OH radicals, which in their turn oxidize the remaining dissolved COD. DOF removes non-biodegradable COD in a high level. As it is obvious in DOF systems, water quality parameters are removed by the two mechanisms of flotation and ozone oxidation. For this the reason, DOF systems have better efficiencies compared to DAF systems. It is estimated that DAF systems will be replaced by DOF systems especially in much polluted water systems to provide a one-step reduction of water quality parameters.
By
Ahmed Ahmed Elserwy
Water & Environmental Consultant
Ain Shames University, Faculty of Science
References
[1] Nicholas P. Cheremisinoff, Handbook of Water and Wastewater Treatment Technologies , Butterworth-Heinemann,2002,p 62.
[2]Lawrence K. Wang, Physicochemical treatment processes, Humana Press Inc, Totowa, New Jersey 0751,2004.
[3] M. Krofta and L. K. Wang, Flotation Engineering. 1st ed. Lenox Institute of Water Technology, Lenox, MA. Technical Manual No. Lenox/1-06-2000/368, Jan. 2000.
[4] 1. G. W. Miller, et al., An Assessment of Ozone and Chlorine Dioxide Technologies for Treatment of Municipal Water Supplies, EPA-600/2-78-147, US Environmental Protection Agency, Cincinnati, OH, 1978.
[5]. NDWC Tech brief: Ozone, Newsletter On Tap, National Drinking Water Clearinghouse (NDWC), No. 12, December, (1999).
[6]. X. Paraskeva, Y. Panagiota, Z. Graham, J. D. Nigel, Water Environment Regulation Watch, November/December, (2002).
[7]. M. R. Collins, Small Systems Water Treatment Technologies: State-of-the-Art Workshop, American Water Works Association, Denver, CO, 1998.
[8]. Tri-State (2003), Ozonation Systems, Energy Technologies, Generation and Transmission Association, http://tristate.apogee.net/et/ewtwozs.asp, November, (2003).