Introduction
Anthropogenic activities include rapidly growing industrialization, a series of new constructions, many fold increases in transportation, aerospace movements, developmental and enhancement in technologies, that is, nuclear power, pharmaceutical, pesticides, herbicides, agriculture, and so on. These are all the most desirable activities for human development and welfare, but they also lead to the generation and release of objectionable materials into the environment. Thus, they pollute the whole environment, making our life on this beautiful earth quite miserable. The situation, if not controlled in a timely manner, would become a malignant problem for the survival of mankind on the earth. Many rivers are being polluted by effluent water from industries and domestic sectors. This creates a problem for the aquatic life by turning water into a resource of no use. So, it is of utmost necessity to solve this problem of water pollution.
The most important challenge in the twenty-first century is to combat against the ever-increasing environmental pollution. To have a neat, clean, healthy, and green environment, there is an urgent need to search for such an approach, which may be applicable at room temperature, safe to handle, economic, and eco-friendly. And above all, the main requirement of the treatment is that it should not be harmful to the environment in any manner.
Although conventional oxidation technologies are available for the oxidation of pollutants or disinfection of pathogenic contaminants using a variety of oxidants such as chlorine, peracetic acid, permanganate, hydrogen peroxide (H2O2), and ozone, there is another group of chemical oxidative processes called advanced oxidation processes (AOPs) or advanced oxidation technologies (AOTs). The concept of AOPs was originally established by Glaze et al. (1987) [1]. It is defined as “oxidation processes, which generate highly reactive radicals (especially hydroxyl radicals) in sufficient quantity to affect the water treatment.” These processes are capable of degrading almost all organic contaminants.
It is clear from standard redox potential data that hydroxyl radical is the strongest known oxidant (2.80 V), second to fluorine (3.03 V).
Therefore, the complete mineralization of most of the organic matters is possible, when the hydroxyl radicals are the main oxidizing species in the solution. This is one of the major advantages of AOPs, since other chemical oxidation processes mostly lead to partial oxidation of the target compounds, and thus, the generation of new hazardous compounds is possible. The other advantage of AOPs is the generation of negligible amounts of residues and their applicability; in case of very low concentrations of pollutants.
The term advanced oxidation processes (AOP), describes a series of processes which are used for the chemical treatment of organic and inorganic pollutants in wastewaters. AOPs are based on the generation of reactive oxygen species (ROS) such as hydroxyl radicals. Generating hydroxyl radicals is possible via various ways such as photocatalytic, electrochemical, sonochemical. Typical AOPs are H2O2/hv, ozone/hv, ozone/ H2O2/hv, TiO2/hv, (photo-)Fenton systems and electrochemical processes.

Advanced Oxidation Processes (AOPs) are efficient methods to remove organic contamination not degradable by means of biological processes. AOPs are a set of processes involving the production of very reactive oxygen species able to destroy a wide range of organic compounds. AOPs are driven by external energy sources such as electric power, ultraviolet radiation (UV) or solar light, so these processes are often more expensive than conventional biological wastewater treatment. Moreover, AOPs can be applied for the disinfection of water, air and for remediation of contaminated soils.
Various AOPs
Although a number of techniques are available under AOPs (more than 10), the main groups of AOPs are four. These are (i) Fenton and photo-Fenton, (ii) ozonolysis, (iii) photocatalysis, and (iv) sonolysis-based processes. These oxidation processes can produce in situ reactive free radicals, mainly hydroxyl radicals. A hydroxyl radical is a nonselective oxidant, which can oxidize a wide range of organic molecules.
A hydroxyl radical has some interesting characteristics, which make it quite important in AOPs. These are:
- It is short-lived
- It can be easily produced
- It is a powerful oxidant
- It is electrophilic in behavior
- It is ubiquitous in nature
- It is highly reactive
- It is nonselective
The reactivity of hydroxyl radical (2.06) is next to that of fluorine (2.23), followed by that of atomic oxygen (1.78), H2O2 (1.31), and then permanganate (1.24). It is the high redox potential of hydroxyl radical that makes it a powerful oxidant. Thus, hydroxyl radicals have emerged not only as an effective but also as an economic and eco-friendly species.
Hydroxyl radicals can react in water by four different routes: (i) addition,(ii) hydrogen abstraction, (iii) electron transfer, and (iv) radical interaction.
The treatment of wastewaters can be carried out using these hydroxyl radicals.
The contaminants are degraded to smaller or less harmful fragments and, in the majority of cases, complete mineralization of the pollutants has been achieved. Even persistent organic pollutants (POPs) can be degraded to the desirable extent using AOPs involving hydroxyl radicals as an active oxidizing agent.
Degradation and detoxification of formalin wastewaters by AOPs has been observed by Kajitvichyanukul et al. (2006) [2]. A comparison of different AOPs for phenol degradation was made by Esplugas et al. (2002). Priya et al. (2008) achieved complete photodegradation of phenol in a reasonable time, that is, less than 5 h, when the concentration of phenol was ≤100 ppm. A comparison of various AOPs has also been given by Saritha et al. (2007) for the degradation of 4-chloro-2-nitro-phenol. The decolorization and mineralization of acid orange-6 azo dye were observed by Hsing et al. (2007) using AOPs. [3,4,5,6]
Kawaguchi (1992) reported the photo oxidation of phenol in aqueous solution in the presence of H2O2. The photo degradation of phenol resulted in the stoichiometric conversion of phenol with practically complete mineralization.
AOP mechanism
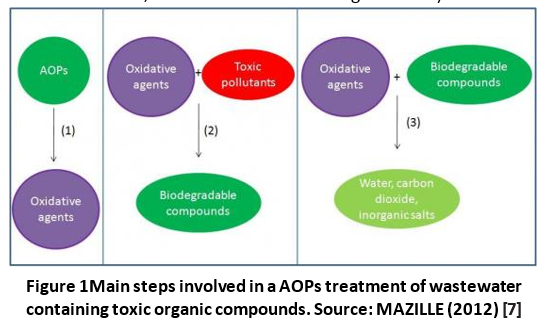
Advanced oxidation involves several steps schematized in the figure below (Figure 1) and explained as follows:
- Formation of strong oxidants (e.g. hydroxyl radicals).
- Reaction of these oxidants with organic compounds in the water producing biodegradable intermediates.
- Reaction of biodegradable intermediates with oxidants referred to as mineralization (i.e. production of water, carbon dioxide and inorganic salts).

By
Ahmed Ahmed Elserwy
Water & Environmental Consultant
Technical Manager Louts for Water Treatment
References
[1] Glaze, W.H., J.W. Kang, and D. Chapin. 1987. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 9: 335–352.
[2] Kajitvichyanukul, P., M.C. Lu, C.-H. Liao, W. Wirojanagud, and T. Koottatep. 2006. Degradation and detoxification of formalin waste water by advanced oxidation processes. J. Hazard. Mater. 135: 337–343.
[3] Esplugas, S., J. Gimenez, S. Contreras, E. Pascual, and M. Rodreguez. 2002. Comparison of different advanced oxidation processes for phenol degredation Water Res. 36: 1034–1042.
[4] Priya, S.S., M. Premalatha, and N. Anantharaman. 2008. Solar photocatalytic treatment of phenolic waste water – Potential, challenges and opportunities. ARPN J. Eng. Appl. Sci. 3(6): 36–41.
[5] Saritha, P., C. Aparna, V. Himabindu, and Y. Anjaneyulu. 2007. Comparison of various advanced oxidation processes for the degradation of 4-chloro-2-nitrophenol. J.Hazard. Mater. 149: 609–614.
[6] Hsing, H.J., P.C. Chiang, E.E. Chan, and M.-Y. Chen. 2007. The decolorization and investigation of acid orange 6 dye in aqueous solution by advanced oxidation processes: A comparative study. J. Hazard. Mater. 141: 8–16.
[7]Mazille, Félicien. “Advanced Oxidation Processes | SSWM. Sustainable Sanitation and Water Management”. Archived from the original on May 28, 2012. Retrieved 13 June 2012.
[8] Comninellis C., Kapalka A., Malato S., Parsons S.A., Poulios I. and Mantzavinos D. (2008) Advanced oxidation processes for water treatment: advances and trends for R&D, J. Chem. Technol. Biotechnol., 83,769-776.