- INTRODUCTION
Aeration brings water and air in close contact in order to remove dissolved gases (such as carbon dioxide) and oxidizes dissolved metals such as iron, hydrogen sulfide, and volatile organic chemicals (VOCs). Aeration is often the first major process at the treatment plant. During aeration, constituents are removed or modified before they can interfere with the treatment processes.
Aeration brings water and air in close contact by exposing drops or thin sheets of water to the air or by introducing small bubbles of air (the smaller the bubble, the better) and letting them rise through the water. The scrubbing process caused by the turbulence of aeration physically removes dissolved gases from solution and allows them to escape into the surrounding air.
Aeration also helps remove dissolved metals through oxidation, the chemical combination of oxygen from the air with certain undesirable metals in the water. Once oxidized, these chemicals fall out of solution and become particles in the water and can be removed by filtration or flotation.
The efficiency of aeration depends on the amount of surface contact between air and water, which is controlled primarily by the size of the water drop or air bubble.
Oxygen is added to water through aeration and can increase the palpability of water by removing the flat taste. The amount of oxygen the water can hold depends primarily on the temperature of the water. (The colder the water, the more oxygen the water can hold).
Water that contains excessive amounts of oxygen can become very corrosive. Excessive oxygen can also cause problems in the treatment plant i.e. air binding of filters.
Water aeration has been long used in water treatment for the removal of odor and taste-causing compounds, the oxidation of iron and manganese, as well as corrosion control and aesthetics. Since the mid-1970s, however, the process has been used to remove carcinogenic and hazardous chemicals from water. These chemicals include volatile organics such as trihalomethanes, radon, trichloroethylene, tetrachloroethylene, 1,1,1-trichloroethane, chloroform, and toluene. As a result, water aeration may be the single most important water treatment process used in the 21st century.
1.1. Types of Aeration Process
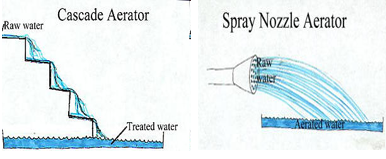
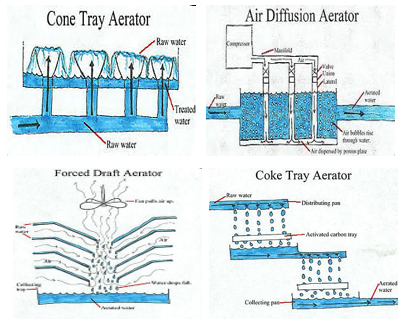
Aeration may be accomplished in a variety of ways using different types of equipment including surface aeration, submerged aeration, and falling water unit [1–6]. The equipment types are listed below:
- Falling Water Units (commonly used in water treatment)
- Spray aerators—water sprayed into the air. Problems include evaporation and freezing.
- Cascade aerators and hydraulic jumps—these operate using waterfalls over a structure.
- Fountain aerators or spray—water cascaded or sprayed over rocks or other types of material.
- Multiple tray aerators with and without coke (often used for iron and manganese removal)—water cascaded over manufactured tray constructed from slats and coke.
- Packed column aeration—air flows up, water is sprayed down (these are efficient and the most common type).


- Surface Aerators (commonly used in the wastewater industry)
- Mechanical surface aerators—water surface is mechanically mixed to increase water to air interface.
- Brush: a series of circular brushes partially submerged are rotated through the water surface to cause turbulence. A support structure is required to suspend the brushes over the water.
- Floating: Floating aerator pumps the water from beneath it up through a draft tube to the surface, which disperses water into the air.
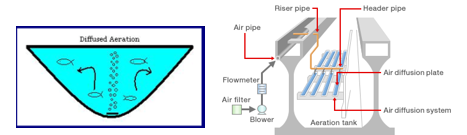
- Submerged Aerators (commonly used in the wastewater and water industries)
- Injection of air with blowers by static tube or diffuser (fine bubble and coarse bubble).
- Jet aeration (the injection of air into pumped water).

- APPLICATIONS
2.1. Taste and Odor Removal
It is sometimes difficult to identify the actual cause of odor and taste problems in water. Some of common odor- and taste-causing compounds include hydrogen sulfide (H2S), methane, algae, oils, phenols, cresols, and volatile compounds. Removal of taste and odor problems is a common application for the water aeration process. The process is suitable for H2S, methane, and volatiles, but not for algae and oils, phenols, and cresols. The compounds must be volatile for aeration to be effective. Aeration is appropriate for many industrial compounds. A classic installation is at Nitro, WV, which utilizes aeration and granular activated carbon (GAC). The raw water had threshold odor numbers (T.O.N.) of 5000–6000 from industrial contamination. The process was effective for reducing the taste and odor down to levels of 10–12 T.O.N. Although taste and odor applications are most common, there are many other tastes and odors that simply cannot be removed by aeration alone, which may explain why so many early plants were abandoned [1–10].
2.2. Iron and Manganese Oxidation
When the total concentration of iron in water is 0.3 mg/L or greater, the iron will cause the water to have an unpleasant taste and redden in color—this may result in the staining of plumbing fixtures and clothes, and accumulations of iron deposits in the water mains. The aeration process is an excellent and common pretreatment application for the removal of iron. First, the process oxides iron by changing the iron from the ferrous state (Fe2+) to the ferric state (Fe3+), which converts the iron from a soluble form (Fe2+) to a non-soluble form (Fe3+) that precipitates from the water. This precipitation
process occurs rapidly when the pH is around 7+. The removal of iron is accomplished through sedimentation and filtration of the precipitated iron. Theoretically, 1 mg/L of O2 will oxidize about 7 mg/L of Fe2+.
Manganese concentrations greater than 0.3 mg/L in water will result in dark brown staining. Oxidation will convert the manganese from Mn2+ to Mn4+ when the pH is above 9. Below a pH of 9, the process is negligibly slow. When tray aerators are utilized for aeration and trays become coated with manganese oxides, the removal is accomplished first by adsorption on accumulated oxidation products (Fe2O3 or MnO2) followed by slow oxidation [2].
2.2.1. Air Injection into Groundwater for Iron Control
In order to lower the iron concentration in groundwater prior to pumping raw water to the municipal water treatment plant, air is injected into the groundwater source. The injected air oxides the iron in the groundwater. This process involves the periodical injection of air into groundwater via a series of wells that surrounds a production well.
This application was implemented in a groundwater supply system in Pembroke, MA.
2.3. Hydrogen Sulfide and Carbon Dioxide Removal
Hydrogen sulfide and carbon dioxide (as carbonic acid and free carbon dioxide) are commonly found in well water. Even a low concentration of hydrogen sulfide can cause odor and taste problems. Hydrogen sulfide is a colorless gas which has a foul odor similar to rotten eggs and is slightly heavier than air (SG = 1.192). In water, molecular hydrogen sulfide is formed from the reduction, dissolves and disassociates in accordance with the reversible ionization reactions:
H2S ↔ HS– + H+
HS– ↔ S2– + H+
The equilibrium of sulfide in water, the percentages of H2S, HS–, and S2– species, is dependent on the pH. Figure 1 shows the distribution of each species at various pH. At a pH of approx 5.7, the sulfide species in water would be near 100% H2S and at approx.
pH 7, 50% of the sulfide species in water would be H2S and the other 50% would be HS– species. The H2S species are volatile; as a result, the aeration process effectively removes it from the water. Therefore, the removal efficiency of sulfide depends on pH.
As the pH increases, aeration becomes less effective because there are fewer sulfides in the form of H2S, which is readily removed by aeration. This process is utilized by both municipalities and chemical industries. In water treatment, the process is called degasification, and is effectively used to remove both H2S and carbon dioxide from well water and product water from the reverse osmosis process.
Dissolved carbon dioxide (CO2) is commonly found in well water. CO2 gas is soluble in water (1700 mg/L @20°C) and is volatile. When CO2 gas is dissolved in water, it forms carbonic acid (H2CO3) and aqueous carbon dioxide (CO2 (aq)). Aqueous carbon dioxide is sometimes identified as free CO2. Using analytical procedures (titration), it is difficult to distinguish between H2CO3 and CO2 (aq); therefore, a hypothetical species (H2CO3*) is used to identify combination of both H2CO3 and CO2 (aq) in water.
H2CO3 is a weak acid. As the water CO2 concentration is increased, then both the H2CO3 concentration and corrosion potential increase. Aeration drives off CO2 and lowers the H2CO3 levels, which reduces the corrosion potential of the water. When both H2S and CO2 are present in water, aeration will remove both. As water is aerated, both CO2 and H2S are removed, but as the pH of the water increases due to the removal of CO2, the removal efficiency of H2S decreases [10].
2.4. Ammonia Removal
This is a limited application in the water industry, but is more commonly used in wastewater treatment. One of the processes utilized in the wastewater industry is the aerated suspended growth process, which utilizes nitrifying bacteria and aeration to convert ammonia to nitrites and nitrates.
2.5. Aesthetic or Decorative Aeration
Fountains are an attractive way to display product water. They can also be functional particularly for taste and odor improvement. Also, they conjure positive associations for many onlookers.
2.6. Reservoir De-stratification and Oxygenation of Water
In small reservoirs and ponds that have trouble maintaining dissolved oxygen levels in water near the bottom of the reservoir (stratification of dissolved oxygen level—high level near the surface and low level near the bottom), aeration can accomplish the following:
it mixes the water, reduces stratification, and increases the dissolved oxygen level in the water. This is accomplished by placing diffusers on the reservoir floor and bubbling air into the water or by using floating aerators. In some cases, this has proved very beneficial, but in other cases it has not been effective; results cannot be determined until the process is implemented. Aeration restores oxygen to water, making the water taste better but it also increases corrosiveness, by increasing the CO2 in the water (resulting from the oxidation of organic matter to CO2). Therefore, there is often a trade off between benefit and detriment [11,20].
2.7. Dissolved Air Flotation for Flocculation/Flotation
Aeration has been used rarely for air flotation for flocculation. The purpose of this application is to increase flocculation size by inducing particle-to-particle contact.
However, air bubbles attached to the flocculation particles often cause them to float rather than to settle. A newer, promising approach is flotation, which injects oxygen saturated water at the bottom of shallow basins, resulting in flocculation forming a scum layer at the water surface, which is then removed [21].
2.8. Trihalomethanes Removal
The aeration process is rated as good to excellent for the removal of trihalomethanes (THM) because they are fairly volatile. This is an increasing application because THMs are not effectively removed by other processes such as granular activated carbon (GAC), although GAC is suitable for organic precursors that react with chlorine to form trihalomethanes.
Aeration is a poor choice for THM precursors removal but suitable for removing trihalomethanes.
2.9. Volatile Organics Removal
The US EPA has identified many types of organic compounds in our water supplies.
Some of the organic compounds are volatile, and, as a result, aeration would be a good process selection for removing them from water. For compounds that are non-volatile, adsorption would be a better process selection than aeration for their removal from the water. Some common volatiles include trihalomethanes, which have already been discussed:
chlorobenzene, 1,1,1-trichloroethane, tetrachloroethylene, and trichloroethylene.
Aeration can achieve up to 95% removal of these compounds.
Non-volatile organic compounds also create problems in water supplies. Adsorption is an excellent removal method for non-volatiles such as styrenes, benzene, phthalates, and fluorine. Therefore, it is often logical to couple air stripping with carbon adsorption, particularly when both volatile and non-volatile compounds are present. Air stripping is particularly suitable for volatile organics because (a) all volatile organics have an affinity for the vapor phase, (b) most organics are hydrophobic—they don’t like water—and (c) air is readily available and inexpensive. Nearly 1000 types of organics have been identified in drinking water and we shall see more applications of air stripping. This will be true particularly in areas of gross contamination.
2.10. Hazardous Waste Cleanup
An increasing amount of contamination results from landfills, leaking containers, and accidental spills. Many of the contaminants are volatile and amenable to aeration. A twofold approach can be used: either clean the water supply or clean the contamination source. When a highly concentrated contaminant is aerated through a packed tower, then air pollution from the aeration process becomes a concern. Air discharge from the packed tower must be collected and treated.
2.11. Radionuclides Removal
Radionuclides are radioactive atoms. The number of protons and neutrons in their nucleus and their energy content are used to characterize the radioactivity of the atoms.
The number following the chemical abbreviation describes the radionuclide composition.
In drinking water, the most commonly found radionuclides are radium, uranium, and radon. Radioactive atoms emit three types of nuclear radiation: alpha, beta, and gamma radiation. Various methods are used to measure the level of radionuclides in drinking water, including counters (internal proportional, end-window, thin window, low-background beta counter, gamma spectrometer, alpha spectrometer, alpha scintillation counter, and liquid beta scintillation counter).
By
Ahmed Ahmed Elserwy
Water & Environmental Consultant
Ain Shames University, Faculty of Science
References
- Great Lakes Upper Mississippi River Board of State Public Health & Environmental Managers (1997) Recommended Standards for Water Works, Health Education Services, A Division of a Health Research, Inc, Albany, NY. www.hes.org.
- L. K. Wang, Personal Communication with New York State Department of Heath, Albany, NY, 2004.
- E. W. Steel and T. J. McGhee,Water Supply and Sewerage,McGraw-Hill Company, NY, 1979.
- US EPA, Technologies for Upgrading Existing or Designing New Drinking Water Facilities, Technology Transfer No. EPA/625/4-89/023, March, US Environmental Protection Agency, Washington, DC, 1990.
- A. Gِkmen, I . G. Gِkmen, and Y. Hung, Radon pollution control, In: Handbook of Environmental Engineering, Volume 2, Air Pollution Control Engineering, (L. K. Wang, N. C. Pereiria, and Hung Y. eds), Humana Press, Inc., Totowa, NJ, 2004. pp. 335–358.
- US EPA, Risk Assessment, Management and Communication of Drinking Water Contamination, Technology Transfer No. EPA/625/4-89/024, April, US Environmental Protection Agency, Washington, DC, 1989.
- US EPA, Innovative and Alternative Technology Assessment Manual, Technology Transfer No. EPA/430/9–78-009, Feb., US Environmental Protection Agency,Washington, DC, 1980.
- Metcalf & Eddy, Inc., Wastewater Engineering Treatment and Reuse, McGraw Hill, Boston, MA, 2003.
- James M. Montgomery, Consulting Engineers, Inc., Water Treatment Principles and Design, John Wiley & Sons, New York, 1985.
- US EPA, Design Manual Odor and Corrosion Control in Sanitary Sewerage Systems and Treatment, EPA/625/1–85/018, Oct., US Environmental Protection Agency, Washington, DC, 1985.
- Jaeger Products, Inc., Air Stripping of VOCs from Water, Brochure Jaeger Products, Inc., www.jaeger.com, 2004.
- J. R. Taricska, Personal Communication with Mufaddal Motiwala of Jaeger Products, Inc., Houston, TX, 2005.
- AWWA, Water Quality & Treatment, 5th ed.,American Water Works Association, McGraw- Hill, Inc., NY, 1999.
- L. K. Wang, J. R. Taricska,Y. T. Hung, J. E. Eldridge, and K. H. Li,Wet and dry scrubbing. In: Handbook of Environmental Engineering, Volume 1, Air Pollution Control Engineering (L. K. Wang, N. C. Pereiria, and Y. T. Hung eds.), Humana Press, Inc., Totowa, NJ, 2004, pp. 197–306.
- Hole Montes, Inc., Pretreatment and Post Treatment Degasifiers and Odor Control Facilities, Technical Memorandum No. 5 Collier County South Water Plant, Florida, 2005.
- J. R. Taricska, Personal communication with Dan Ching of Met Pro/Duall Division, Owosso, MI, 2004.
- J. R. Taricska, Personal communication with Paul Jacobs of TSC-Jacobs, Tampa Florida, 2005.
- National Drinking Water Clearinghouse, Organic Removal, Tech Brief, Aug, 1997.
- G. R. Scott, J. AWWA 32(9), 873, 1955.
- S. I. Roth, Noise Level Measurements and Recommendations–Degasifier Fans and Odor Control Fans, Collier County South Water Plant, Florida, 2004.
- L. K. Wang, E. Fahey and Z. Wu. Dissolved air flotation. In: Handbook of Environmental Engineering, Volume 3, Physicochemical Treatment Processes. (L. K. Wang, Y. T. Hung and N. K. Shammas eds.). Humana Press, Inc. Totowa, NJ 2005, pp. 431–500.