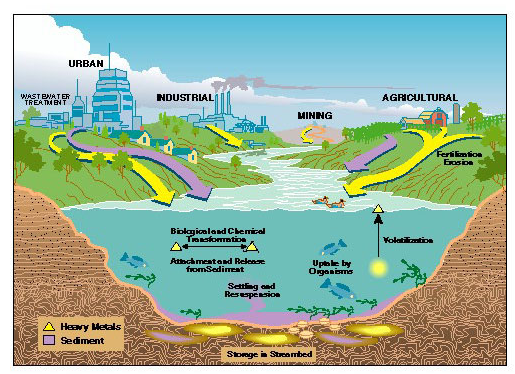
Every day each one of us needs around 1.8 to 2.0 liters of water with the purpose of keeping in fine fettle body. Peoples who drink very little, water they drink a variety of drinks made from water such as tea, juice, some of these drinks contains very high concentrations of aluminum, polycyclic aromatic hydrocarbons (PAHs) and fluoride as well as high concentration of several other metals and compounds. (Brönmark, et.al 2017).
Generally heavy metals cause a harmful effect for humans and aquaculture. As an example, lead is very toxic to living organisms because it accumulates in the bones, brain, kidney and muscles. Heavy metals may be the reason of many serious disorders such as kidney diseases, nervous disorders and even death. (Pandey, et.al 2014).
One of the most toxic elements of heavy metals is Cadmium. Even at low concentration, it can accumulate in the food chain and reach the human body. The metal accumulates in the liver and kidneys and causes damage if the exposure is chronic. Also, it has been considered the main reason of itai-itai disease in Japan. (Santoyo-Sánchez, et.al 2018).
Cadmium
Cadmium is not important for biological systems and has been reported to have dangerous effects. It is used in the manufacturing industries such as in batteries and pigments. (Nwose, et.al 2015).
Phosphate fertilizers are routes for cadmium transference into the environment. Cadmium is highly toxic to human beings and animals at very low concentrations; Smoking of cigarettes is one of the main sources of cadmium poisoning. (Dixit, et.al 2015).
Chromium
Chromium is used in a wide range of industrial applications, such in leather, tanning, paper industries and rubber engineering. High levels of exposure have harmful effects on central nervous system and cause liver and kidney damage. Also Chromium can reduce the photosynthesis rate in plant species; It was also linked to the toxic effects on immune response in freshwater fish. (Prozialeck, et.al 2016).
Copper
Copper has been used for a long time during old history in utensils production, wires, pipes and bronze alloy manufacturing. It has a good role as an essential element for human and animal bodies. However higher dose shows toxic effects, such as on kidneys, stomach damage, vomiting and loss of strength. (Manahan, 2017).
Arsenic
Arsenic naturally aggravated by over powering aquifers and from phosphorus fertilizers industries. High arsenic concentrations in water can have bad effect on the health. Arsenic concentrations are more than the acceptable limits in water have bad effect on the human, animal, plant health. During 2012, in West Bengal arsenic was found in high concentrations in drinking water in several areas. Most of the people in these areas suffering from arsenic skin lesions. It was proved that arsenic contamination in the groundwater have negatively health effect. (Rose, 2017).
Aluminium
Aluminium in detectable amounts can occur in many natural water resources due to incomplete removal during the treatment process in drinking water plants or as a result of sub strata leaching. (Hassan, 2017).
The usual source of aluminium in public water supplies is aluminum salt in coagulation process. According WHO Guidelines the recommend aluminium concentrations should not more than 0.2 mg Al /L in the water. Aluminium is a mandatory parameter in the new UK Regulations. (Helz, 2018)
Iron
Iron is a common metal found in most natural water resources. Iron also can be found in several forms, as a colloid or as visible particles; or as a complex with organic substances or other minerals (Yu, et.al, 2017).
Hematite {Fe2O3}; pyrite{Fe2S2}; ilmenite{FeTiO3}; magnetite{Fe3O4}; siderite{Fe2CO3} and limonite {FeO(OH)} are the principal ores of iron. (Larsen, 2012)
In surface waters Iron is usually in the ferric form(Fe3+), but (Fe2+) ferrous the more soluble form is likely to be present in deoxygenated conditions which mostly may occur in groundwater or in the waters of reservoirs and lakes bottoms. By exposure to air water rapidly become discolored because the iron oxidizes to the ferric form (Fe+3) and precipitates out. (Bratby, 2016).
Iron salts as ferric chloride (FeCl3) and Ferrous Sulphate (FeSo4) for many years are used as coagulants extensively in water treatment. With good process control, it be possible to keep the residual iron concentration less than 0.05 mg/L in the inlet of water unit. Incomplete removal of iron during treatment process can lead to deposits of iron in the distribution system over a period of time. (Sharma, 2014).
The iron concentrations found in drinking water are not harmful but can cause a bad taste when present more than 1 mg/l. At lower iron concentrations it may cause ‘dirty’ water problems, with consumer rejection to this water appearance. It can also have formed brown stains on laundry and plumbing fittings. The WHO guideline value is not more than 0.3 mg/l to avoid discoloration and staining. Iron is an important parameter in the EC Directive, with a value 200 µg/l. This is matching the standard of current UK regulations.
Manganese
Manganese can be found in both surface and ground waters in detectable concentrations. The concentration of manganese in ground waters is much higher concentrations than in surface water subject to anaerobic conditions. Manganese from the bottom sediments can dissolve in the bottom water when becomes deoxygenated in impounding reservoirs. This leads to increase of the overall manganese concentration of the water. Manganese is toxic when be large concentrations. (Sharma, 2014)
Manganese is undesirable in drinking water, even in small concentrations, in the presence of oxygen; manganese can precipitate from water also after chlorination. Deposition of manganese is generally lower than iron in a distribution system. In 2008, the WHO has set a guideline value for manganese of 0.4 mg/l on health grounds (Sharma, 2014).
but recommended concentration on the basis of staining laundry is lower than 0.1 mg/l. (Tekerlekopoulou et.al, 2013)
Iron and manganese can be found at high concentrations in some water sources. When the water is aerated, Iron and manganese are oxidized to their oxides which are low in solubility. This push consumer to search for to alternative supplies ((Sharma, 2014).
Exceeding the recommended maximum contaminant levels causes discoloured water, undesired taste; laundry problem as yellow points, and low flow from water lines. This in turn, results dissatisfaction of consumer with the water utility. (Sharma, 2014).
High concentrations of manganese mainly affect the brain tract. Poisoning with manganese cause hallucinations, forgetfulness, nerve atrophy. (Sharma, 2014).
Before iron and manganese removal from water by any method, some chemical change must take place. Chemical changes are also usually responsible for any obstacles in removing Fe and Mn. (Sharma, 2014).
By
Ahmed Hasham
M.Sc. Env. Analytical chemistry
ORCID: 0000-0002-0202-6664
References:
Brönmark, C., & Hansson, L. A. (2017). The biology of lakes and ponds. Oxford University Press.
Bratby, J. (2016). Coagulation and flocculation in water and wastewater treatment. IWA publishing.
Dixit, S., Yadav, A., Dwivedi, P. D., & Das, M. (2015). Toxic hazards of leather industry and technologies to combat threat: a review. Journal of Cleaner Production, 87, 39-49. doi.org/10.1016/j.jclepro.2014.10.017.
Hassan, R. A. M. (2017). Effect of Aluminum Chloro Hydrate Treatment on the Physiochemical Properties of River Nile Water at Omdurman, Sudan (Doctoral dissertation, University of Gezira). http://repo.uofg.edu.sd/handle/123456789/808
Helz, G. R. (2018). Aquatic and Surface Photochemistry: 0. CRC Press.
Larsen, G. R. (2012). Determination of coastal ground and surface water processes and character by use of hydrochemistry and stable isotopes, Fraser Coast, Queensland (Doctoral dissertation, Queensland University of Technology).
Manahan, S. (2017). Environmental chemistry. CRC press.
Nwose, C., Nwachukwu, K. C., & Okoh, M. P. (2015). Food chain exposure to heavy metals (cadmium and arsenic), effects on Plasma, tissue triglyceride concentration, experimental rats, a model study. IJCS, 3(2), 102-108.
Pandey, G., & Madhuri, S. (2014). Heavy metals causing toxicity in animals and fishes. Research Journal of Animal, Veterinary and Fishery Sciences, 2(2), 17-23.
Prozialeck, W. C., Lamar, P. C., & Edwards, J. R. (2016). Effects of sub-chronic Cd exposure on levels of copper, selenium, zinc, iron and other essential metals in rat renal cortex. Toxicology reports, 3, 740-746. doi.org/10.1016/j.toxrep.2016.09.005.
Rose, J. G. (2017). Legal Foundations of Environmental Planning: Textbook-casebook and materials on environmental law. Routledge.
Santoyo-Sánchez, M., Thévenod, F., & Barbier, O. (2018). Cadmium Handling, Toxicity and Molecular Targets Involved during Pregnancy: Lessons from Experimental Models. Metal Metabolism in Animals, 18, 272. doi:10.3390/ijms18071590.
Yu, J., Han, Y., Li, Y., Gao, P., & Sun, Y. (2017). Separation and recovery of iron from a low-grade carbonate-bearing iron ore using magnetizing roasting followed by magnetic separation. Separation Science and Technology, 52(10), 1768-1774. doi.org/10.1080/01496395.2017.1296867