Introduction
The incomplete application of Quality Assurance system inside wastewater laboratories will lead to obtain inaccurate and unreliable laboratories results , and these inaccurate results will lead to incorrect decisions that will affect the proficiency of wastewater treatment plants and affect the treatment operations parameters of the plant , therefore obtain treated water which not compatible to environmental laws and regulations. So applying Quality Assurance system inside laboratory is very important for obtaining accurate and reliable analytical results.
Implementation of the Quality Assurance system inside laboratory is considered to be one of the major standards that all wastewater laboratories should be done. Application of Quality assurance system in laboratories improves the quality of test results and elevates the confidence level from the customer side.
The laboratory Quality Management System will consist of Quality assurance manual, written procedures, work instructions, and records. The manual should include a quality policy that defines a statistical level of confidence used to express the precision and accuracy of data .Quality systems, which include quality assurance policies and all quality control processes, must be in place to document and ensure the quality of analytical data produced by the laboratory and to demonstrate the competence of the laboratory. (1)
Quality Assurance is the definitive program for laboratory operation that specifies the measures required to produce defensible data of known precision and accuracy. This program will be defined in a documented laboratory quality system. (1)
Also Quality Assurance can be defined as it is a Part of quality management focused on providing confidence that quality requirements will be fulfilled. (2)
Quality Assurance objectives
It is the responsibility of the laboratory’s management to identify and state, in writing, what the quality goals of the laboratory are to be.
The primary objective of a laboratory’s quality assurance system is to improve and maintain at a high level the precision and accuracy of the laboratory’s “product.” Here, the laboratory’s product can be defined as “the report issued as the result of analytical, measurement, or testing activity conducted on a sample or samples received from some source.” Management, administrative, statistical, investigative, preventive, and corrective techniques are among those which may be used to maximize the quality of the reported data. (3)
Secondary objectives which may be established to reach primary goals might be:
- To establish the level of the laboratory’s routine performance.
- To make any changes in the routine methodology found necessary to make it supportive of the management policy regarding reduction of costs associated with corrective action and evaluation.
- To set objectives associated with achieving management’s mandate to assure continuous improvement of quality performance.
- To establish program goals for the laboratory’s quality and technology training efforts. (3)
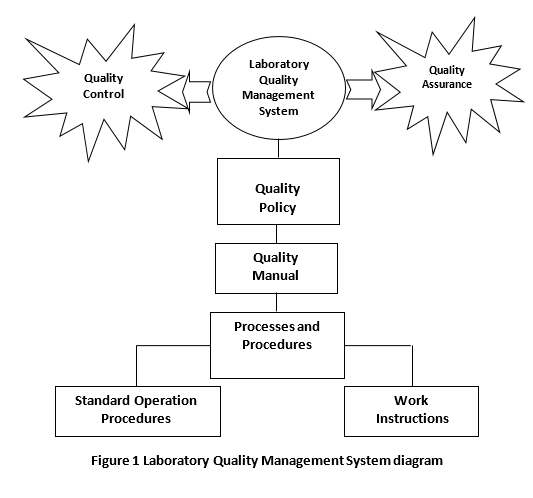
As shown in figure 1 laboratory quality management systems based mainly on quality control activities and quality assurance, and consists of Quality assurance manual, written procedures, work instructions, and records. Quality system of laboratory includes a quality policy of organization that provides guidance to the organization on the pathway to continuous improvement of its quality performance.
Stages of Establishment of Quality Assurance system inside wastewater laboratories
The quality assurance system of wastewater laboratories is based according to requirements of Standard Methods for the Examination of Water and Wastewater 20th Edition.
Since 1905, Standard Methods for the Examination of Water and Wastewater has represented “the best current practice of American and European water and wastewater analysts.
The 20th Edition of “Standard Methods for the Examination of Water and Wastewater are approved by USA Protection Agency’s (EPA) Serving as a comprehensive guide for water and wastewater quality testing of a wide variety of contaminants in water and wastewater. This comprehensive reference covers the following:
- All aspects of water and wastewater analysis techniques.
- All Quality Control measures and activities inside water and wastewater laboratories.
- All Quality Assurance procedures applied in water and wastewater laboratories for assure quality and accuracy of analysis results.
1- First stage Studying the Laboratory Current Situation
Laboratory made analytical study for all data and information specified to laboratory current situation to know the exact status of laboratory and evaluation the possibilities and available means for applying quality assurance system laboratory.
Laboratory made a comparison between requirements of Standard Methods for the Examination of Water and Wastewater and the actual practices done in the laboratory which is already applied in current situation. Gap analysis is done between standard requirements and available possibilities for determine the additional work should be done to start applying QA system as well as efforts required for maintain laboratory efficiency.
Gap analysis results state that laboratory needs more efforts to improve quality like:
- Lab staffs need more training about Quality control of analytical results.
- Lab did not have standard procedures for sampling, calibration and equipment maintenance.
- Lab sampling staffs need training for proper collection and handling of samples.
2– Second Stage Enlightenment, and Training of lab staff
All laboratory staff is subjected to training courses and practices which concentrate on Enlighten and inform them clearly the importance, goals, objectives and benefits of applying quality assurance system inside laboratory.
The following Training courses had been done internally for lab staff:
- Gable wastewater treatment principals and techniques.
- Sampling quality control.
- Quality control for wastewater laboratories.
- Quality assurance for wastewater laboratories.
All training courses and practices are designed well to ensure that all laboratory staff is aware with all necessary knowledge and information related to quality system that affect their duties and tasks .after training the courses and practices are reviewed and evaluated to determine the degree of awareness and benefits.
3- Third stage Writing all procedures, work instructions and documents
Procedures, documents and instructions of sampling, analytical methods, instrument calibration, equipments maintenance, chemicals handling, storage, data reduction, validation and reporting, quality control and corrective, preventive actions had been written for preparing quality manual.
4- Fourth stage Writing Quality Assurance manual
By assistance of consultants Quality manual is determined and written which include all definitions and concepts of quality assurance and also include quality policies of the laboratory. Quality manual refers to all procedures, instruction, actions and polices that laboratory follow to fulfill the managerial and technical requirements of standard method.
Steps of Preparation for Write Quality Assurance Manual.
Preparation and writing Quality Assurance Manual of Gable laboratory processed the following steps inside laboratory:
- All technical and administrative aspects inside laboratory are organized and arranged.
- Importance, goals, objectives and benefits of applying quality assurance are determined and identified to all laboratory staff.
- Establishing Objectives and Priorities
- Collection and Review of Existing Procedures.
- Preparation of a Flowchart.
- Identification of Program Requirements.
- Identification of Shortfalls and the Assignment of Priorities.
- Writing the Manual.
- Reviewing and making necessary changes.
In the laboratory quality assurance manual all managerial responsibility, authority, quality goals, objectives and commitment to quality are specified and documented clearly.
The manual is written clearly to ensure that all laboratories personal understand their roles and responsibilities.
Procedures are instituted to permit tracing a sample and its derivatives through all steps from collection through analysis to reporting final results to the laboratory’s client and disposal of the sample .Adequate and complete documentation are performed to assure data defensibility and to meet laboratory qualification requirements and ensure full traceability for all tests and samples.
Preventive maintenance procedures are used and documented for instrumentation and equipment to reduce instrument malfunction, maintain more consistent calibration, be cost effective, and reduce downtime. Measurement traceability is included to standard reference materials in the Quality Assurance manual or Standard Operation Procedure to establish the integrity of the laboratory calibration and measurement program.
Logbooks are maintained for each test or procedure performed with complete documentation on preparation and analysis for each sample, including sample identification , associated standard and QC samples , method reference ,date/time of preparation/analysis ,analyst, weights and volumes used, results obtained and any problems encountered. Logbooks are kept that document maintenance and calibration for each instrument or piece of equipment. The data obtained from an analytical instrument are subjected to data reduction processes described in the applicable SOP before the final result can be obtained. Calculations and any correction factors, as well as the steps to be followed in generation the sample result are specified in the quality assurance manual or SOP. Analytical results are reported in standard units of mass, volume, or concentration as specified in the method of analysis or SOP.
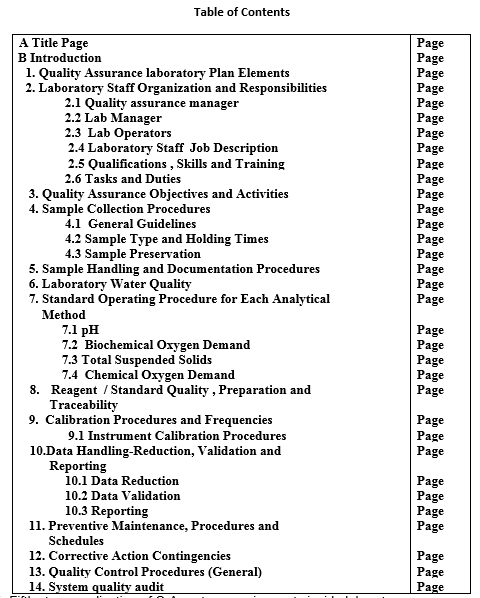
The following table shows components of Gable wastewater laboratory Quality Assurance Manual.
5– Fifth stage application of Q.A system requirements inside laboratory
Applications of Q.A system requirements according to quality plan are done by make and activate all procedures and work instruction which cover all laboratory processes and activities. Laboratory processes and activities include all laboratory files such as Standard Operation Procedures SOPs file, Instruments and Equipment’s file, Personal file, and Documents and Records files.
6- Sixth Stage Review (Auditing) of laboratory Quality System
Auditing is processed to determine whether the quality activities and their results comply with the established documentation; to confirm whether these activities are appropriate for achieving the objectives proposed and whether they have been implemented effectively.
Review System Efficiency has been done by detecting the nonconformity points for system requirements. Internal auditing is performed by Quality Assurance department. Internal auditing has been achieved through all laboratory regulations and through standard quality techniques and done for self-evaluation and for improvement.
6- Seventh stage Corrective and Preventive Actions.
Corrective and Preventive Actions are executed to ensure achieving the goals of applying quality assurance system followed by monitor and control the execution of these actions.
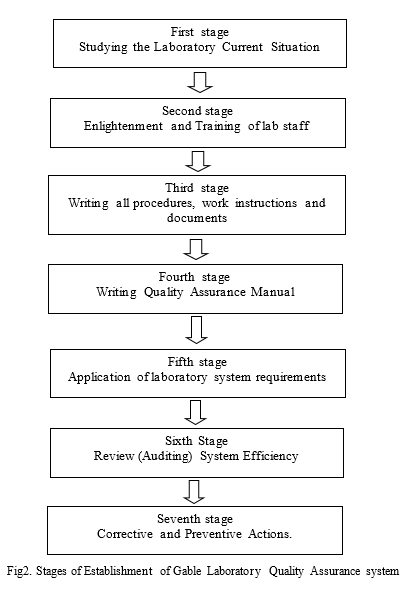
The following figure shows the stages of Establishment of Quality Assurance system inside Gable laboratory.
By
Ahmed Ahmed Elserwy
Water & Environmental Consultant
Technical Manager Louts for Water Treatment
References
(1) APHA, AWWA, and WEF, 2005. Standard Methods for the Examination of Water and Wastewater, Twentieth Edition, Washington, DC,2003.
(2) AOAC International (2006), Terms and Definitions. Available: www.aoac.org/terms.htm accessed 23 September 2006.
(3) Thomas A. Ratliff, The Laboratory Quality Assurance system, John Wiley & Sons, Inc. 2003