Selected Nanomaterials applications in waste water treatment
Ahmed Hasham (M.Sc. Env. Chemistry) / Ahmedhasham83@outlook.com
Introduction:
Water pollution is the most difficult environmental challenges facing society. Therefore, the release of pollutants from sewage or industrial wastewater in the must be considered as a threat to the environment. Characterizing the physical, chemical and biological aspects of raw water and treated water is crucial to ensure that they are safe for disposal in the aquatic or desert environment.
Water pollution:
Leaching of pollutants into groundwater from sewage or industrial water cause serious health problems, which may be used by humans for drinking and other purposes in some areas. It is worth mentioning that a man cannot live more than three days without water.
Heavy metals are likely to be the most common water problem consumers face. Heavy metals (such as arsenic, zinc, iron, manganese, aluminum, cadmium, lead, etc.) cause many health problems if found in drinking water at concentrations higher than permitted.
Nanotechnology history:
Nanotechnology first appeared millions of years ago as molecules began to arrange in complex shapes and structures that launched life on Earth. At the nanoscale, substances have different physical, chemical and biological properties than their normal size characteristics.
Nanomaterials
Nanomaterials may be defined as materials smaller than 100 nm in at least one dimension. At this scale, materials regularly have novel size-dependent properties different from their large scale.
Nano-materials has the ability to clean up huge polluted locations, saving time, excluding the need for removal of pollutants, and hence decreasing pollutant concentration to the minimum levels. Advances in nanoscale science suggest that many of the current problems on water quality could be solved or avoided by using nanomaterials such as non-adsorbent, nanocatalyst, bioactive nanoparticles, nanostructured catalytic membranes, nano-powder, nanotubes, magnetic nanoparticles, nanosensors. Nanomaterials are the main players that promise many profits through their nanoenabled applications in multiple fields. Nanomaterials has been used in many environmental applications such as the treatment of contaminated water for drinking, agriculture and more recent application than through conventional means. The explosive development in nanotechnology research has presented new strategies in environmental remediation.
Some of these applications using the nanomaterials properties that relate to their high specific surface area, such as fast dissolution, high reactivity, and strong sorption. Others take advantage of their discontinuous properties, such as superparamagnetic, localized surface plasmon resonance (SPR), and quantum confinement effect. Most applications discussed in this article are still in the stage of research and development.
Titanium dioxide nanoparticles
Titanium dioxide nanoparticles, also called ultrafine titanium dioxide, are particles of titanium dioxide (TiO2) with diameters less than 100 nm. Ultrafine TiO2 is used in sunscreens due to its ability to block UV radiation while remaining transparent on the skin, and its photocatalytic sterilizing properties also make it useful for many applications.
Nano particles of TiO2 are found different in their surface-to-volume ratio, their properties change so that they acquire catalytic ability. Activated by the ultraviolet (UV) component in sunlight, they break down toxins or enhance other relevant reactions. Titanium oxide photocatalysts have been broadly studied for solar energy conversion and environmental applications in the past several decades, because of their high chemical stability, good photoactivity, relatively low cost, and nontoxicity.
Photocatalytic oxidation process:
In the photocatalytic oxidation process, organic pollutants are cracked in the presence of semiconductor photocatalysts, an energetic light source, or an oxidant such as oxygen or air. Only photons with energies greater than the band gap energy (ΔE) can result in the excitation of valence band (VB) electrons that then promote possible reactions.
Recently, advanced oxidation processes (AOPs) using (TiO2) have been used successfully to toxic pollutants removal from industrial wastewater.
TiO2 features as photocatalytic agent:
- High photochemical reactivity.
- High photocatalytic activity.
- Low cost.
- Stability in aquatic systems.
- Low environmental toxicity.
Mechanism of dye degradation upon irradiation
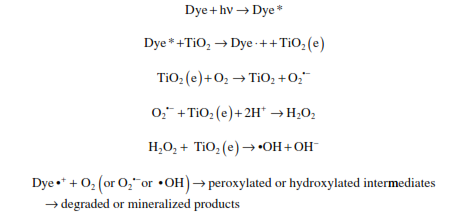
Photocatalytic oxidation is an AOP for elimination of trace pollutants and microbial pathogens. It is a valuable pretreatment for hazardous and non biodegradable pollutants to enhance their biodegradability. Photocatalysis can also be used as a polishing step to treat recalcitrant organic compounds. The major barrier for its wide application is slow kinetics, due to limited light fluence and photocatalytic activity. Current research focuses on increasing photocatalytic reaction kinetics and photoactivity range.
Gold nanoparticles
The modification of the Au surface with appropriate chemical species can improve separation and preconcentration efficiency, analytical selectivity; make the Gold nanoparticles (AuNPs), is consider the one of the wide selections of core resources available, coupled with tunable surface properties in the form of inorganics or inorganic–organic amalgams, have been described as an excellent platform for a broad range of analytical methods.
Advantage of Gold nanoparticles (AuNPs):
- High surface-to-volume ratio.
- Easy surface modification.
- Simple synthesis methods.
The AuNPs have been applied successfully used in:
- Removal of peptides.
- Removal of proteins.
- Removal of heavy metal ions.
- Removal of polycyclic aromatic hydrocarbons (PAHs).
Zerovalent iron nanoparticles
Elemental iron has been used as an ideal candidate for remediation, for the following reasons:
- Low-cost,
- Extremely easy to prepare and apply to a variety of systems.
- No toxicity induced by its usage.
The idea of using metals such as iron as remediation is depend on reduction–oxidation reactions, in which a neutral electron donor (a metal) chemically reduces an electron acceptor (a pollutant). Nanoscale iron particles have surface areas greater than larger-sized powders, which leads to enhanced reactivity for the redox process.
Iron nanoparticles applications such as:
- Decomposition of halogenated hydrocarbons to benign hydrocarbons.
- Remediation of heavy metals.
- Solvents dechlorination
A significant loss of reactivity can occur before the particles are able to reach the target contaminant. In addition, zerovalent iron nanoparticles tend to flocculate when added to water, resulting in a reduction in the effective surface area of the metal.
Therefore, the effectiveness of a remediation depends on the accessibility of the contaminants to the nanoparticles, and the maximum efficiency of remediation will be achieved only if the metal nanoparticles can effectively migrate without oxidation to the contaminant or the water– contaminant interface.
To overcome such difficulties, a regularly used strategy is to integrate iron nanoparticles within support materials, such as polymers, porous carbon, and polyelectrolytes.
Finally, Nanotechnology for water and wastewater management is depending on the matchless properties of nanomaterials and their conjunction with current treatment technologies present great chances to revolutionize water and wastewater treatment. Nanotechnology has shown huge possibility in water treatment technologies. The recent development of nanotechnology has raised the possibility of environmental decontamination through several nanomaterials cut the process and tools.
Another nanomaterials application in water treatment field may be discussed in another article.
للاطلاع على أكبر مكتبة مجانية في مجال علوم وهندسة المياه يرجى زيارة المنتدى من الرابط التالي
watertechexperts.com/vb/forum.php
للاطلاع على مزيد من المقالات باللغة الانجليزية يرجى زيارة مدونتنا باللغة الانجليزية من الرابط التالي
www.water-tech-market.com/blog
لارسال أي استفسارات أو أسئلة أو طلبات يرجى الانضمام لجروب المنتدى على الفيس بوك من الرابط التالي
www.facebook.com/groups/waterexperts
References
Abhijith, K.S., and Thakur, M.S. Analytical Methods, 2012, 4, 4250–4256.
Cao, G.Z. Nanostructures and Nanomaterials, Synthesis, Properties and Application, Imperial College Press,
London, 329, 2004.
Chae, H.K., Perez, D.Y.S., Kim, J., Go, Y., Eddaoudi, M., Matzger, A.J., O’Keeffe, M., and Yaghi, O.M. A route
to high surface area, porosity and inclusion of large molecules in crystals. Nature, 2004, 427, 523–525.
Chen, L., Lou, T., Yu, C., and Kang, Q. N-1-(2-mercaptoethyl)thymine modification of gold nanoparticles: A highly selective and sensitive colorimetric chemosensor for Hg
. Analyst, 2011, 136, 4770–4773.
Chun, C.L., Penn, R.L., and Arnold, W.A. Environmental Science and Technology 2006, 40, 3299–3304.
Cloete, T.E., Kwaadsteniet, M.D., Botes, M., and Lopez-Romero, J.M., Nanotechnology in Water Treatment Applications. Caister Academic Press, Wymondham, UK, 2010.
Elimelech, M., and Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science, 2011, 333, 712.Eshel, K. British Medical Journal, 2007, 334, 610–616.
Furukawa, H., Cordova, K.E., O’Keeffe, M., and Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science, 2013, 341, 1230–1234.
Guzman, K.A.D., Taylor, M.R., and Banfield, J.F. Environmental Science and Technology, 2006, 40, 1401–1407.
Hang, Y., Qin, Y., and Shen, J. Separation and microcolumn preconcentration of traces of rare earth elements on nanoscale TiO2 and their determination in geological samples by ICP-AES, Journal of SeparationScience, 2003, 26, 957–960.
Harada, M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Critical Reviews in Toxicology, 1995, 25, 1–24.
Hidaka, H., Jou, H., Nohara, K., and Zhao, J. Photocatalytic degradation of the hydrophobic pesticidePermethrin in fluoro surfactant/TiO2 aqueous dispersions. Chemosphere, 1992, 25, 1589–1597