Introduction
Coagulation is an essential phenomenon in industrial wastewater treatment. Inorganic coagulants (salts of multivalent metals) are being commonly used due to its low cost and ease of use. However, their application is constrained with low flocculating efficiency and the presence of residue metal concentration in the treated water.
Organic polymeric flocculants are widely used nowadays due to its remarkable ability to flocculate efficiently with low dosage.
Inorganic Coagulants
Inorganic salts of multivalent metals such as alum, poly aluminum chloride, ferric chloride, ferrous sulphate, calcium chloride and magnesium chloride have been widely used for decades as coagulant.
Since the effect of coagulant is considerably influenced by the application conditions, such as water pH and stirring condition.
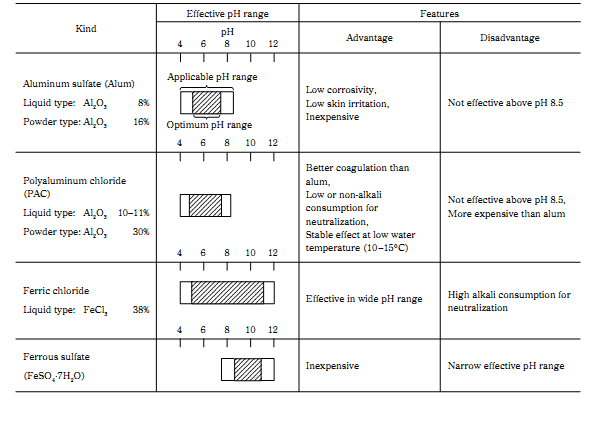
– pH
Each inorganic coagulant has the optimum pH, this optimum pH range is the same to the pH range which the metallic ion composed of the coagulant stably precipitates as the hydroxide. For example, aluminum ion stably precipitates as the hydroxide in the pH range of 5.0 to 7.5, and the optimum pH range of alum or PAC is also 5.0 to 7.5.
This fact shows that not only electrical charge neutralization but also formation of metal hydroxide floc plays the important role for the better coagulation of particles with inorganic coagulants.
– Stirring strength and retention time
Strong stirring is required to increase the frequency which coagulants meet suspended particles to neutralize their electrical charges. Stirring also accelerates the floc formation by colliding the neutralized particles.
In the case of mixing tank for coagulation, the blade tip velocity of stirrer and the retention time are generally designed in the range of 1.5 to 3.0 m/s and 5 to 10 minutes respectively.
In case that water to be treated includes stable emulsifying particles, etc., the stirring strength and retention time should be strengthened and extended.
– Order of chemical injection
Chemicals using for coagulation and flocculation treatment should be injected into the water in the order of inorganic coagulant, pH control agent (alkali) and polymer flocculent.
If alkali is added prior to coagulant, the charge neutralization ability of coagulant may be deteriorated because the coagulant will deposited as the metal hydroxide in water soon after the injection.
Organic Coagulants
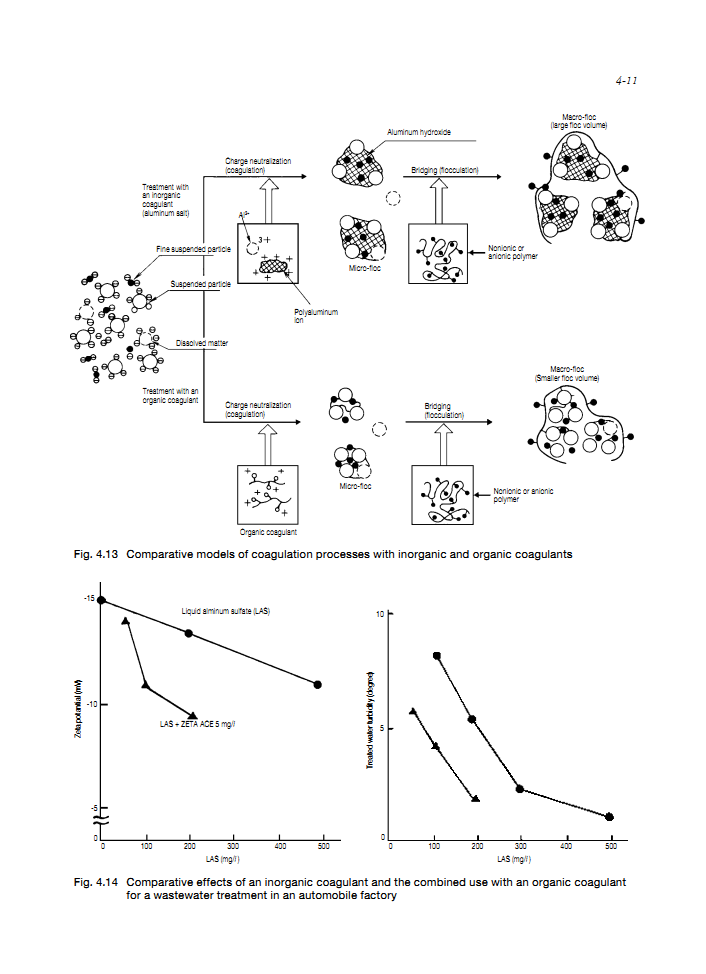
The coagulation effect of organic coagulants is superior to the inorganic coagulants because of the higher ionic valences.
Some organic coagulants not only neutralize the charge of SS but also react with dissolved anionic organic compounds, such as lignin-sulfonate, anionic-surfactant, arginic acid and humic acid, and form the water insoluble salts.
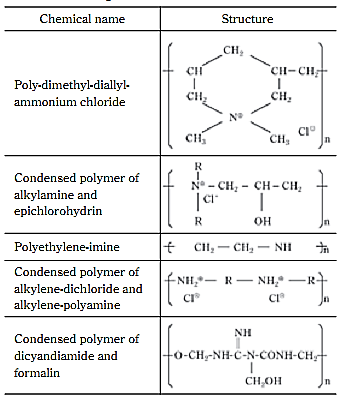
Most organic coagulants are cationic low molecular weight polymers, their cationic functional groups are essentially amine-groups including primary, secondary and tertiary amine-groups, and quaternary ammonium chloride groups.
Organic coagulants show the excellent charge neutralizing abilities, however, they hardly form flocs by themselves.
Generally, the combined use of organic coagulants together with inorganic coagulants shows the better coagulation effect.
comparison between inorganic and organic coagulants
inorganic Organic
• cheap
• Production of a lot of sludge
• high dosages (100 – 500ppm)
• Efficiency depends on the pH
• Increase in salinity
• corrosive ions (Cl–and SO42–) concentration in treated water • Low dosages (10 – 50ppm)
• Effective at a high range of pH
• Reduction of the sludge production
• Better in applications with oil, fats and or surfactants
• No increase of salinity
Ahmed Amer
Oil field services Engineer