1.Introduction
In 1748, the French physicist Nollet first noted that water would diffuse through a pig bladder membrane into alcohol. This was the discovery of osmosis, a process in which water from a dilute solution will naturally pass through a porous membrane into a concentrate solution. Over the years, scientists have attempted to develop membranes that would be useful in industrial processes, but it wasn’t until the late 1950s that membranes were produced that could be used for what is known as reverse osmosis. In reverse osmosis, water is forced to move through a membrane from a concentrate solution to a dilute solution.
Since that time, continual improvements and new developments have been made in membrane technology, resulting in ever-increasing uses in many industries. In potable water treatment, membranes have been used for desalinization, removal of dissolved inorganic and organic chemicals, water softening, and removal of the fine solids.
In particular, membrane technology enables some water systems having contaminated water sources to meet new, more stringent regulations. In some cases, it can also allow secondary sources, such as brackish groundwater, to be used. There is great potential for the continuing wide use of membrane filtration processes in potable water treatment, especially as technology improves and costs are reduced.

Membrane separation systems have many advantages over traditional water or wastewater treatment processes, including [1–12]:
- Fewer chemicals are used in the process, which helps minimize the negative impacts of those chemicals on the whole process.
- Formation of absolute barriers to particle and pathogens. Microorganisms such as bacteria and viruses can be removed by size exclusion; ultra-pure water can therefore be produced.
- Lower operating and maintenance costs in comparison to conventional systems consisting of coagulation, clarification, and aerobic and anaerobic treatments.
- Membrane separation systems are easy to operate and the performance is more reliable.
- Membrane systems give a compact and modular construction, which occupies less floor space in comparison to the conventional treatment systems. This becomes extremely attractive in the land-scarce countries such as Japan and Singapore.
- Membrane systems followed by an evaporator (for low-volume highly concentrated effluent) can enable industries to achieve zero liquid discharge goals.
- One-stop reduction or elimination of most contaminants (impurities) in the wastewater stream, e.g., total dissolved solids (TDS), chemical oxygen demand (COD), 5-d biochemical oxygen demand (BOD5), total organic carbon (TOC), color, suspended solids, nitrogen, phosphorus, and heavy metals.
- Permeate can be suitably reused resulting in water conservation, which reduces the intake
of raw water and provides savings on raw water processing costs.

- Membrane Filtration
A membrane is a thin layer of semi-permeable material that separates substances when a driving force is applied across the membrane. Membrane processes are increasingly used for removal of bacteria, microorganisms, particulates, and natural organic material, which can impart color, tastes, and odors to water and react with disinfectants to form disinfection byproducts.
- Description of Membrane Filtration Processes
In the simplest membrane processes, water is forced through a porous membrane under pressure, while suspended solid, large molecules or ions are held back or rejected.
- Types of Membrane Filtration Processes
The two general classes of membrane processes, based on the driving force used to make the process work, are:
- Pressure-driven processes
- Electric-driven processes
4-1.Pressure-Driven Processes
The four general membrane processes that operate by applying pressure to the raw water are:
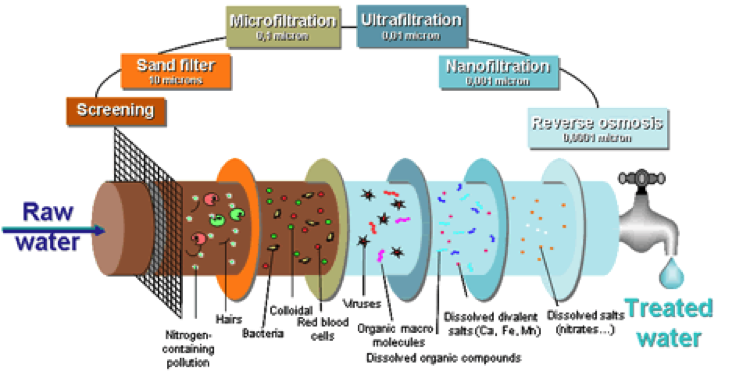
- Microfiltration
- Ultrafiltration
- Nanofiltration
- Reverse Osmosis
Microfiltration
Microfiltration (MF) is a process in which water is forced under pressure through a porous membrane. Membranes with a pore size of ranging from 0.1 to 10.0 um are normally used; this size is relatively large compared with the other membrane filtration processes. This process has not been generally applicable to drinking water treatment because it either does not remove substances that require removal from potable water, or the problem substances can be removed more economically using other processes. The current primary use of MF is by industries to remove very fine particles from process water, such as in electronic manufacturing. In addition, the process has also been used as a pretreatment for other membrane processes. In particular, RO membranes are susceptible to clogging or filter binding unless the water being processed is already quite clean.
However, in recent years, microfiltration has been proposed as a filtering method for particles resulting from the direct filtration process. Traditionally, this direct filtration process has used the injection of coagulants such as alum or polymers into the raw water stream to remove turbidity such as clay or silts. The formed particles were then removed by rapid sand filters. The use of filter aids to improve filtering efficiency, especially for small particles that could contain bacterial and protozoan life are recommended.
Ultrafiltration
Ultrafiltration (UF) is a process that uses a membrane with a pore size generally below 0.1 um. The smaller pore size is designed to remove colloids and substances that have larger molecules, which are called high-molecular-weight materials. UF membranes can be designed to pass material that weigh less than or equal to a certain molecular weight.
This weight is called the molecular weight cutoff (MWC) of the membrane. Although UF does not generally work well for removal of salt or dissolved solids, it can be used effectively for removal or most organic chemicals.
Most UF membranes are made from polymeric materials, such as, polysulfone, polypropylene, nylon 6, PTFE, polyvinyl chloride, and acrylic copolymer. Inorganic materials such as ceramics, carbon-based membranes, and zirconia, have been commercialized by several vendors.
Applications of UF process for water and wastewater treatment include:
- Oil emulsion waste treatment
- Production of ultra-pure water for electronics industry
- Reduction of high COD levels in corn starch plants
- Selective removal of dissolved toxic metals from groundwater in combination with chemical treatment
- Treatment of whey in dairy industries
- Wine or fruit juice clarification
Nanofiltration
Nanofiltration (NF) is a process using membranes that will reject even smaller molecules that UF. The process has been used primarily for water softening and reduction of total dissolved solids (TDS). NF operates with less pressure that reverses osmosis and is still able to remove a significant proportion of inorganic and organic molecules. This capability will undoubtedly increase the use of NF for potable water treatment. NF membranes can be used to produce drinking water, because they have good molecular rejection characteristics for divalent cations, e.g., calcium and magnesium.
Membranes used for NF are made of cellulose acetate and aromatic polyamide with characteristics such as salt rejections from 95% for divalent salts to 40% for monovalent salts and an approximate MWCO of 300 for organics. An advantage of NF over RO is that NF can typically operate at higher recoveries, thereby conserving total water usage due to a lower concentrate stream flow rate. NF is not effective on small-molecular weight organics, such as methanol.
Reverse Osmosis
Reverse Osmosis (RO) is a membrane process that has the highest rejection capability of all the membrane processes. These RO membranes have very low MWC pore size that can reject ions at very high rates, including chloride and sodium. Water from this process is very pure due to the high reject rates. The process has been used primarily in the water industry for desalinization of seawater because the capital and operating costs are competitive with other processes for this service. The RO also works most organic chemicals, and radionuclides and microorganisms. Industrial water uses such as semiconductor manufacturing is also an important RO process.
Almost all RO membranes are made of polymers, cellulosic acetate and matic polyamide types, and are rated at 96–99% NaCl rejection. RO membranes are generally of two types, asymmetric or skinned membranes and thin film composite membranes. The support material is commonly polysulfones, while the thin film is made from various types of polyamines and polyureas.

Reverse osmosis membranes have been used widely for water treatment such as ultrapure water makeup, pure boiler water makeup in industrial fields, seawater and brackish water desalination in drinking water production, and wastewater treatment and reuse in industrial,
agricultural, and indirect drinking water production as shown in Table 1.
Table 1. Application of reverse osmosis membrane process [13]
| Industrial use | Drinking water | Wastewater treatment and reuse |
| Ultrapure water, boiler water, process pure water, daily industries. | Sea water desalination ,brackish water desalination | Industrial water, agriculture water, indirect Drinking water |
Other common applications of RO include:
- Desalination of seawater and brackish water for potable use. This is very common in coastal areas and. the Middle East where supply of fresh water is scarce.
- Generation of ultrapure water for the microelectronics industry.
- Generation of high-purity water for pharmaceuticals.
- Generation of process water for beverages (fruit juices, bottled water, beer).
- Processing of dairy products.
- Concentration of corn sweeteners.
- Waste treatment for the recovery of process materials such as metals for the metal finishing industries, and dyes used in the manufacture of textiles.
8. Water reclamation of municipal and industrial wastewaters

4-2.Electric-Driven Processes
There are two membrane processes that purify a water stream by using an electric current to move ions across a membrane. These processes are:
- Electrodialysis
- Electrodialysis reversal
Electrodialysis
Electrodialysis (ED) is a process in which ions are transferred through a membrane as a result of direct electric current applied to the solution. The current carries the ions through a membrane from the less concentrated solution to the more concentrated one.

Electrodialysis Reversal
Electrodialysis Reversal (EDR) is a process similar to ED, except that the polarity of the direct current is periodically reversed. The reversal in polarity reverses the flow of ions between demineralizing compartments, which provides automatic flushing of scale forming materials from the membrane surface. As a result, EDR can often be used with little or no pretreatment of feed water to prevent fouling. So far, ED and EDR have been used at only a few locations for drinking water treatment.

By
Ahmed Ahmed Elserwy
Water & Environmental Consultant
Ain Shames University, Faculty of Science
References
- H. S. Ong, Challenges ahead for Singapore’s water supply. Seminar on Ensuring Singapore’s Water Supply: Options and Issues, Shangri-la Hotel, 10–11 Nov. (1997).
- M. Joel, E. O. Peter, and R. W. Mark, Water Treatment Membrane Process, McGraw-Hill Company, New York, 1996, pp. 17.1–17.31.
- S. Judd and B. Jefferson (eds.), Membrane for Industrial Wastewater Recovery and Re-use, Elsevier Advanced Technology, Oxford, 2003.
- J. P. Chen, S. L. Kim, and Y. P. Ting, Optimization of feed pretreatment for membrane filtration of secondary effluent. Journal of Membrane Science 219, 27–45 (2003).
- S. L. Kim, J. P. Chen, and Y. P. Ting, Study on feed pretreatment for membrane filtration of secondary effluent. Separation & Purification Technology 29, 171–179, 2002.
- R. D. Letterman (ed.), Water Quality and Treatment, A Handbook of Community Water Supplies, 5th ed., McGraw-Hill, New York, 1999.
- W. S. W. Ho and K. K. Sirkar (eds.),Membrane Handbook, Chapman & Hall,New York, 1992.
- Singapore Public Utilities Board. Singapore Water Reclamation Study, Expert Panel Review and Findings. Singapore (2002).
- T. Matsuura, Progress in membrane science and technology for seawater desalination—a review. Desalination 134, 47–54 (2001).
- T. Matsuura, Synthetic Membranes and Membrane Separation Processes, CRC Press, Boca Raton, FL, 1994.
- T. Matsuura and S. Sourirajan, Studies on reverse osmosis for water pollution control. Water Research 6, 1073–1086 (1972).
- Metcalf and Eddy, Inc. (ed.), Wastewater Engineering: Treatment Disposal and Reuse, 4th ed., McGraw-Hill, New York, 2002.
- Norman N. Li, Anthony G. Fane, W. S. Winston Ho, and T. Matsuura, Advanced Membrane Technology and Applications, 2008 John Wiley & Sons, Inc.